Colours of Ancient Egypt – Green
By Anna Pokorska, on 6 March 2019
This is the fifth post in the Colours of Ancient Egypt series; you can read the introduction here, or all about the colour blue, red, and yellow.
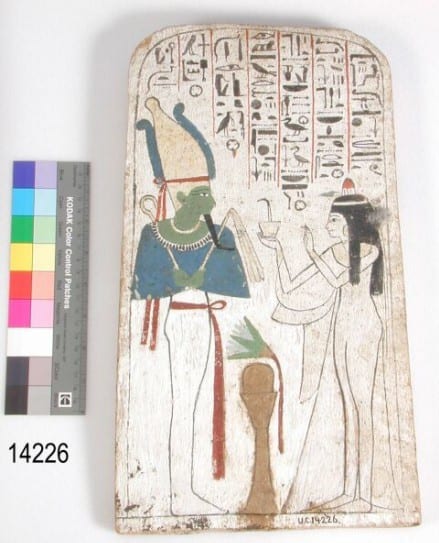
In Ancient Egypt, perhaps unsurprisingly, the colour green was associated with life and vegetation. However, it was also linked with the ideas of death. In fact, Osiris, the Egyptian god of fertility, death and afterlife, was commonly portrayed as having green skin. Even scarabs, popular amulets and seals, were often green due the beetle’s symbolic connotation to rebirth and immortality.
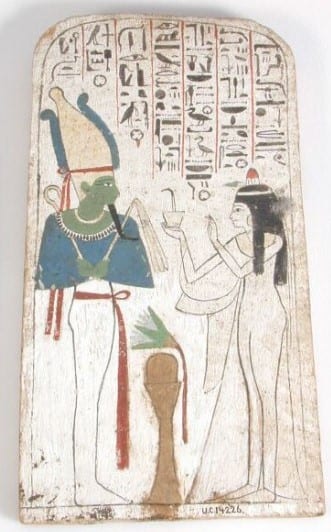
Painted wooden stela of Neskhons, wife of the High Priest of Amun Pinedjem (II) making an offering to Osiris, identifiable by his green skin (Petrie Museum, UC14226).
By far the most prevalent, and likely the oldest, green pigment was made from a mineral called malachite. It is a copper carbonate and a relatively stable colourant, although sensitive to excessive heat and acid exposure. It was popular in Egyptian tomb painting from the 4th Dynasty (c. 2613 to 2494 BC) onwards but didn’t find much use in European painting until the 15th and 16th centuries.

Cross-section of malachite (Image: Rob Lavinsky).
A copper acetate, called verdigris, has also been found on Egyptian art. It gives a slightly transparent bluish green, often applied over a ground of lead white or lead-tin yellow. It’s artificially produced by exposing copper plates to acetic acid, a by-product of wine-making. The reaction that follows produces a blue-green deposit, which can then be scraped off, ground, and used as a pigment. Unfortunately, verdigris is very reactive and can become dark brown or even black with ageing. However, it was identified as the primary green pigment on the headband of Queen Nefertiti’s bust, where it retains its hue.
In addition to its instability, verdigris is also moderately toxic due to its copper content. Therefore, its use gradually declined through history, to be mostly replaced by a new pigment, viridian, developed and patented in France in 1859. Viridian is both permanent and non-toxic which immediately made it a great substitute for the older green pigments.

The famous bust of Queen Nefertiti on display at the Neues Museum, Berlin (Image: Philip Pikart).
Other sources of green colour included an artificial green frit (produced the same way as blue frit or Egyptian blue, except that the lime content has to be higher than the copper content) as well as mixing Egyptian blue with yellow ochre. The latter method was occasionally used during the 12th Dynasty (1991-1786 BC) but became popular during the Amarna period (1370-1352). For faience, copper and iron oxides were mostly used, until the discovery of yellow lead antimonate gave Egyptian artisans many more choices of hue.
Green was certainly a colour of great importance to Egyptians, although nowadays it appears overshadowed by the significance and properties of Egyptian blue. However, we can still find and admire green pigments in great condition amongst ancient Egyptian artefacts. Next time you’re visiting the Petrie Museum, check out the wall block fragment from the pyramid of King Pepy I with instructions on his ascent into heaven! Guess what colour the inscription is…;)
 Close
Close