Simulating nanocomposites
By Oli Usher, on 15 December 2014

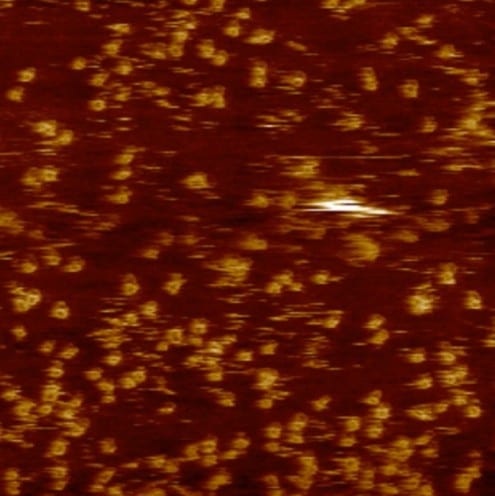
A long chain-like molecule of polyvinyl alcohol (in red) interacting with the tiny sheets that make up a clay particle. Top left: after 0.15 nanoseconds, top right: after 0.8ns, bottom left: after 2.5ns, bottom right: after 4.75ns. Credit: Advanced Materials/Suter/Coveney/Groen
Supercomputer simulations carried out by UCL chemists have successfully modelled the behaviour of clay-polymer nanocomposite materials. These materials, produced by dispersing tiny particles of clay through polymer (plastic) are widely used in industry.
Clay particles are made of tiny mineral sheets; plastics are made of long chain-like molecules. The way these interact when they are mixed determines the properties of the resulting composite material.
In the simulation, images of which are shown above, the polymer chains do not only surround the clay particles, they actually slide into the gaps between the sheets that make them up.
Read a full report of the science behind these pictures on the UCL Mathematical & Physical Sciences website, or read the coverage of the breakthrough on BBC News.
Links
High resolution image
UCL stars on ‘The Sky at Night’
By Oli Usher, on 15 December 2014
December’s Sky at Night was practically a UCL full house.
As well as Maggie Aderin-Pocock (UCL Physics & Astronomy) presenting, the programme featured UCL astronomers Serena Viti and Steve Fossey, UCL chemists Andrea Sella and Stephen Price, and was filmed at UCL’s observatory.
Well worth a watch – viewers in the UK can watch the programme again on BBC iPlayer until 14 January 2015 by clicking above.
The show will also be repeated on BBC Four at the following times: 7.30pm on 18 December, and 2am on 19 December.
PanCam taking shape
By Oli Usher, on 8 December 2014
UCL’s contribution to the ExoMars Rover – the European Mars mission scheduled for touchdown in 2019 – is gradually taking shape. Quite literally.
This photo shows a prototype of the main structure of the panoramic camera (PanCam) instrument being machined out of a block of metal. PanCam will be the rover’s primary camera, producing high resolution 3D images of the Martian surface, and it is being being built by scientists and engineers at UCL’s Mullard Space Science Laboratory.
The structure is known as the ‘optical bench’. All the precision-built optical components, including detectors, lenses, mirrors and filter wheels (below), will be attached to this rigid housing.
Links
High resolution images
10 Questions
By ucqndko, on 5 December 2014
An interview in 10 Questions…
In this monthly feature, the Institute of Biomedical Engineering (IBME) interviews our researchers, academics, students, clinicians, affiliates and partners to find out a little more about who they are and what they do.
This month the IBME interviewed Dr Ben Hanson, Senior Lecturer in the Department of Mechanical Engineering, UCL

1. What is your job title?
Senior Lecturer & Departmental Tutor
2. How long have you worked as a Senior Lecturer & Departmental Tutor?
Nine years as a Lecturer, appointed as Departmental Tutor last year.
3. What keywords would you pick to describe your work?
Biomechanics, signal processing.
4. What is your favourite thing about your work?
Solving problems, that’s what engineering is all about. It’s lovely to work in hospitals, and with inspiring people who make a real difference to people’s lives. We’re doing a great project at the moment showing scary movies to people with implanted pacemakers in an attempt to identify how stress affects the heart. [UCL News; Time Magazine]
5. What’s been your career highlight?
My first publication in a medical research journal, (Circulation, 2009). That took a lot of hard work and head-scratching, but my struggle to understand the workings of the heart and to make sense of some data we’d recorded turned out to be really useful in revealing how things worked at a fundamental level.
6. What is your favourite quote?
Miss Piggy: “Never eat more than you can lift” (a great image and, I guess, a lesson in biomechanics).
7. Who has been your greatest mentor and why?
My first PhD supervisor, Andy Plummer, who is such a nice human being as well as being an engineering genius: he demonstrated that the two are compatible.
8. What do you do in your spare time?
Eat! Often with friends & family, often something I’ve made: I am a chocoholic and like making chocolates. My research involves the biomechanics of eating and drinking for people who have difficulties, e.g. due to a stroke, surgery or dementia. I’m very motivated to find ways to control the mechanical properties of foods & drinks to make them easier to manage so that I can continue to enjoy my food when I’m old & frail.
9. What’s your favourite book at the moment?
I was laughing out loud at “The hundred-year-old man who climbed out of the window and disappeared”.
10. If you had a superpower, what would it be and why?
I love it when the clocks go back and you have an extra hour in the day so I would have an extra hour please, then I might get to do some of the things that unfortunately get squeezed out.
Dr Ben Hanson is a Senior Lecturer in the Department of Mechanical Engineering at University College London, with Affiliate status in the Division of Medicine. Dr Hanson researches biomedical applications of engineering, system-modelling and analysis.
Nanodrills in action
By Oli Usher, on 2 December 2014
A team of scientists at UCL and partner institutions has today published a study showing how certain harmful bacteria use tiny ‘nanodrills’ to make holes in our cell membranes.
These rings of toxin molecules assemble themselves on the cell membrane, then slice down, punching a hole and spitting out the piece of membrane they cut away. The rings then hold the hole open, much like an eyelet.
This image is a still from a ‘video’ produced by an atomic force microscope (AFM) in Bart Hoogenboom’s lab at UCL. AFMs feel a surface rather than seeing it – a tiny needle is repeatedly moved across the surface and feels the shape and hardness of the sample: lighter colours represent raised surfaces.
In the full video (below), we see the ring-like structures skating over the surface of the membrane, before they start perforating the membrane.
Links
High resolution images
Humans on the surface of Mars by the 2030s
By ucassya, on 25 November 2014

NASA chief scientist Ellen Stofan speaks at UCL. Credit: Satureyes Photography (All rights reserved)
NASA has set its sights on expanding our presence into the Solar System by developing the capabilities needed for humans to step foot on Mars for the first time in the 2030s. This is the message of NASA’s Chief Scientist Dr Ellen Stofan and Chief Technologist Dr David Miller, outlined in a recent public lecture they gave at UCL.
NASA is currently working with 16 space agencies around the globe and the U.S. commercial space industry as part of a Global Exploration Roadmap to make the exploration of Mars a reality.
Future human exploration could answer one of the most fundamental unanswered questions: Is it possible that life can exist beyond Earth?
This journey begins in low-Earth orbit on the International Space Station where groundbreaking science takes place to develop the technology and communication systems that are essential for survival in space. Scientific advances are also being made in understanding how the body changes in microgravity and the impact the space environment has on mental health.
For over 40 years we have already landed robotic spacecraft on the surface of Mars. With the Curiosity rover discovering evidence that water persisted on the surface of Mars for millions of years is it possible to demonstrate life once existed? The rover has also measured the radiation levels that humans will be exposed to during the duration of the mission, helping in the development of their protection.
Why stop at Mars? Where else can we go? We have already observed water plumes erupting from the South Pole of Jupiter’s moon Europa and flew the Cassini spacecraft through ice plumes shooting from the surface of Saturn’s moon Enceladus.
With missions such as Kepler detecting over 2500 exoplanet candidates in our region of the Milky Way, surely we aren’t alone?
- Stephanie Yardley is a PhD student at UCL Mullard Space Science Laboratory
Peeling an egg
By Oli Usher, on 24 November 2014
This sequence of images shows the painstaking act of peeling a frog’s egg. The egg is gripped with tweezers, carefully torn open, and the cell nucleus inside is separated out. The nucleus is the small, pale blob in the centre of the final frame, and it is well under a millimetre across.
This difficult procedure is a key element in new research published by UCL scientists today. In the study, scientists probed the membrane that surrounds the cell nucleus in order to determine the structure of tiny pores that play a key role in cell biology.
A full summary of their research, including a remarkable image of these pores, produced using an atomic force microscope, is available from UCL News.
You can also watch a video of the process below.
Links
High resolution images
Charged with uncertainty: how a classic classroom experiment reveals what we don’t know about static electricity
By Oli Usher, on 20 November 2014
Rub a balloon or a plastic rod, charging it up with static electricity, and it can suddenly pick up little pieces of paper. It’s a common classroom demonstration in high school science classes, an everyday example of electrostatic attraction. But it’s never explained fully in class – pupils are told about plastics gaining or losing electrons and becoming charged, but why (and how) this charge is actually created is never explained.

Static electricity picking up scraps of paper. Photo: Tess Watson (CC BY)
The reason for this is as simple as it is surprising: nobody actually knows.
***
Much of what we are taught in school science lessons is wrong. Electrons are not billiard balls spinning around an atomic nucleus – even if that’s a useful metaphor. Newton’s laws do not govern the motion of objects – though in most cases, they are so close as makes no difference. Copernicus did not claim, let alone prove, that the Earth orbits the Sun, though his model of the cosmos was an important innovation in the history of astronomy.
In all these cases, the classroom version is a reasonable approximation, a simplification which still helps children learn about science, without getting bogged down in unnecessary complexity.
But in the case of plastic being charged up with static electricity, scientists genuinely don’t fully understand what’s going on.
***
Katherine Holt is a researcher in UCL Chemistry who has worked on electrostatic charging, and is hoping to answer some of these questions. Initially interested in the way that charge can build up on the surfaces of nanodiamonds (microscopic diamonds formed in the soot created by explosives), she has come to work on the question of how static electricity forms on the surfaces of plastics.
For many years, this was the preserve of physicists, who looked at the big picture of how charge behaves on the surface of a whole object. But with the improvement in tools such as magnetic force microscopes, the field has in recent years opened up to chemists, who are interested in small-scale properties of materials, such as bonds between atoms, breaking and joining of atoms in molecules, the microscopic structure of surfaces and the behaviour of individual ions and electrons.
This change of emphasis, Holt says, has brought about a growing conviction that even the most superficial explanation given in school (“electrons are transferred to or from the plastic when it is rubbed”) is wrong. It is increasingly clear that the charging process is actually linked to imperceptible damage to the surface. Bonds between atoms on the plastic’s surface are being broken by the rubbing and this – not the exchange of electrons between surfaces – is leading to the charge being created.

Tunneling electron microscope image of two polystyrene beads rubbing together and then separating that clearly shows that material is transfered from one surface to another as they do so and that the surfaces become ‘scarred’ and damaged. Credit: Katherine Holt (UCL Chemistry)
But identifying microscopic damage on the surface and linking that to the creation of static electricity isn’t the same as figuring out what is actually going on.
***
When you break bonds between atoms in molecules, shattered fragments of those molecules (either individual atoms or groups of atoms) are formed. These highly reactive particles are known as free radicals.
And indeed, Holt says, you can find free radicals on the surface of charged plastic rods. What’s more, in the same location as these radicals, surface imaging techniques reveal the presence of electric charge.
But even this still doesn’t solve the mystery of precisely how electrostatic charging works: radicals are electrically neutral, so some other particle – presumably ions or electrons – must be carrying the charge.
There is more that isn’t known.
For instance, whether a plastic will become positively or negatively charged is easily predicted, there is even a table (the triboelectric series) which sets out which combinations of common polymers will charge each other positively or negatively when rubbed against each other. But the triboelectric series is based on experimental data – there is no underlying theory explaining what is actually going on, and Holt says, there may never be one: there could be a diverse range of different phenomena all contributing to the different electrostatic properties of the different plastics.
***
Working in a field which is experimentally well understood, but lacking in theoretical explanation, is not an entirely comfortable area for scientists. For engineers, however, who are interested in practical applications and less worried about theory, this is far more usual territory.
Holt says that, despite being a chemist, she’s now finding it easier to talk to engineers about her work in this area than she does with colleagues in closely aligned fields of chemistry. Engineers are interested in electrostatic charging for prosaic reasons – preventing powders from clumping together in factories, for example – and have an extensive practical understanding of it. But they don’t care in what’s happening on a molecular level.
Which, when the underlying science is as intractable as this, seems like quite a tempting thing to do.
Related links
Using phones to prevent pandemics
By Oli Usher, on 18 November 2014
Rachel McKendry (London Centre for Nanotechnology at UCL) leads a consortium that develops mobile phone-based diagnostic kits. She gave this year’s Rosalind Franklin lecture at the Royal Society, which you can view above.
In this lecture, Professor Rachel McKendry presented her research to create a new generation of mobile phone connected diagnostic tests for infectious diseases. The widespread use of mobile phones could dramatically increase access to testing outside of hospital settings, particularly in developing countries. Professor McKendry also presented the research foundations of a global early-warning system for infectious diseases that links the millions of symptoms that are self-reported on the web each day to mobile phone connected tests, in real-time and with geographically-linked information. This research lies at the cutting edge of infectious diseases, nanotechnology, telecommunications, big data and public health.
Links
Fossil crab
By Oli Usher, on 17 November 2014
This specimen, from UCL’s Geology Collections, shows a well-preserved fossilised crab. Its legs are largely intact and even the texture of its abdomen can be made out. The claws, however, are missing.
Crabs’ claws are one way to tell male and female specimens apart (males’ claws are generally larger). Interestingly, the shape of a crab’s underside also hints at its sex in most species.
(Any amateur or expert determinations of this crab’s sex are most welcome in the comments below.)
Crabs have existed since the Jurassic period, 145-200 million years ago.
 Close
Close