Why is the universe speeding up?
By Oli Usher, on 27 October 2015
The Dark Energy Survey aims to answer that question, and UCL astrophysicists are heavily involved in the collaboration.
This film explains the project – and features Prof Ofer Lahav (UCL Physics & Astronomy), one of the leading figures of the DES science programme.
Solar scientist sculpted
By Oli Usher, on 21 October 2015
The Royal Society has recently unveiled a bust of Lucie Green to join their collection of eminent scientists.
Lucie is Professor of Physics at UCL’s Mullard Space Science Laboratory, an expert on the sun’s magnetic field and frequently appears on TV as a presenter or guest on space and astronomy programmes.
This is all maths. Honest.
By ucahpse, on 12 October 2015
On the Sunday and Monday of the August Bank Holiday, UCL held its inaugural Spark Festival at the Olympic Park in Stratford. Filled with stalls representing research from across the STEM subjects (Science, Technology, Engineering and Maths), it was designed to inspire children to become the scientists of tomorrow.
Seven mathematics PhD students – Anna Lambert, Huda Ramli, Matthew Scroggs, Matthew Wright, Oliver Southwick, Pietro Servini and Rafael Prieto Curiel – went along to see whether they could set up some experiments…
In 2012, athletes from across the world dived into the 50 m long swimming pool nestled within the streamlined Aquatic Centre of Stratford’s Olympic Park. The British charge, led largely by Rebecca Adlington, stuttered and sank, with only three medals and none of them gold.
But even they have an easier time swimming than the smallest organisms that inhabit our planet, for whom swimming in water is exactly like us trying to move in corn syrup. Hence their development of long tails (or flagella) that they move in a corkscrew movement in order to propel themselves forwards: were they simply to flick their tails one way and then the other, they would end up right back where they started, never going anywhere.
Not the best way to win in the race called life.
But why swim in a liquid in the first place, when you could just run across it?
We’re mathematicians! We don’t really do experiments – unless you count the simulations we run on computers or the thought processes we carry out in the giant hallways of our minds. For sure, we don’t spend our days in the office filling up inflatable paddling pools with dozens of litres of water and a hundred kilos of cornflour, going slowly crazy as we croak out “Corn!” in the manner of Lord Mormont’s raven in Game of Thrones. Which means that we’re not very good when it comes to optimising the area around the pool that should be covered with a tarpaulin, especially when there are kids around. Or, for that matter, figuring out how to pipette food colouring into a cylinder full of corn syrup – probably the stickiest substance known to humanity[citation needed] – and then take out the pipette without removing the food colouring too, when the whole purpose of the experiment is to show that the flow is reversible and that that is exactly what will happen.
But how else were we to entertain the hundreds of children who attended UCL’s Spark Festival at the Olympic Park over the August Bank Holiday weekend, a two day extravaganza of stalls showcasing an eclectic collection of research being done in the STEM subjects?
And so there we were, surrounded (hopefully) by the young scientists of the future, who were excitedly mixing vials (plastic cups) of sticky food dye together; transferring the semi-solid, semi-liquid mixture of cornflour and water from one bucket to another (and, obviously, to the ground as well); excitedly jumping up and down on the surface of this non-Newtonian fluid in our paddling pool (or falling down in it); whilst we were trying desperately to convince them and their parents that this was indeed all maths.
Which, of course, it is. Our mixture of cornflour and water (also known as oobleck) is an example of a non-Newtonian fluid: where the viscosity (the fluid’s “thickness”) is not constant as it is in air or water, but either increases (as in our case) or decreases (paint, ketchup, toothpaste, blood) as you apply a force to it. The study of these substances is important (or so we tell ourselves) and mathematicians play a key role in modelling their flow to predict what they might do in certain situations.
Shear thickening substances such as ours, for example, are being used to develop the body armour of the future.
Our Taylor-Couette experiment involved a highly viscous fluid (corn syrup) filling the space between an inner rotating cylinder and an outer cylindrical wall and some blobs of differently coloured food dye placed within the gooiness. When the fluid is rotated, the food colouring is mixed together; but rotate now in the opposite direction and the indistinct mess separates out into the original distinct blobs. We have reversible flow, where time doesn’t really matter and frictional forces dominate; and all because the Reynolds number,
is very low. In our Taylor-Couette experiment, this was because the viscosity was very high; but mathematicians use the same equations to model the flow of lava (also high viscosity) or the motion of glaciers (low typical speed) or, yes, the swimming of microbes in water (small length scale).
So how much did we succeed in proving that splashing around in oobleck or getting covered in sticky corn syrup was maths? Who knows! But hopefully we planted that seed of thought that maths is more than what you study in school; that it’s exciting, fun and beautiful; that it can provide stunning insights into the mysteries of nature; and that it can link together things that seem completely different. And at least we succeeding in helping to ensure, as one young girl breathlessly shouted as she frantically tried to stay afloat on our cornflour, that this engineering festival was “way better than Imperial’s!”
Even if we did have to spend Tuesday morning on our hands and knees, scrubbing the ground with a broken broom and chipping away at an inch-thick layer of cornflour on the grass, cursing our inability to properly site a tarpaulin.
Total eclipse of the Moon
By Oli Usher, on 28 September 2015
Last night saw both a supermoon (the Moon’s closest approach to Earth, in which it appears about 14% bigger than it does at its most distant), and a lunar eclipse, in which the full Moon passes through the Earth’s shadow.
During a lunar eclipse, the disc of the Moon progressively goes from bright white to a deep red: when in the Earth’s shadow, the only light illuminating its surface is the light that is bent through Earth’s atmosphere. This light – effectively, the light of all the sunrises and sunsets on Earth – is red because blue light is scattered in Earth’s atmosphere.
This sequence of photos was produced by Theo Schlichter, Computing and Instrumentation Officer at UCL’s observatory, using a Canon EOS450D camera and a 200mm lens. The composite was produced by Dr Steve Fossey.
First person: 8.3 magnitude earthquake hits Chile
By ucapola, on 23 September 2015
A combination of high mountains, clear skies and bone-dry deserts makes the north of Chile one of the world’s best places to observe the sky. Numerous international observatories are located there, and astronomers from around the world frequently travel there to carry out their research. Ofer Lahav, Perren Professor of Astronomy at UCL, was there during the magnitude 8.3 earthquake of 16 September. This is his personal account of the events.
The Earthquake started on Wed 16 Sep at 19:54 (local time), just as we were preparing for the start of DES (Dark Energy Survey) observations.
We left the telescope immediately, and moved to the shaky ground outside the dome. Eventually we were evacuated from there to the CTIO dining hall.
After-shocks continued throughout the night, but the oscillations finally decayed.
The following morning was sunny and quiet. Observations resumed the following night.

Boulders dislodged from the mountainside and fell onto the road near the observatory. Photo: Ofer Lahav
I left next morning as planned, flights were on schedule.
We were all impressed by the way the observatory staff handled the situation efficiently and calmly.
It is re-assuring that the system, in part assembled at UCL, is working so well – the only obstacles between the DECam instrument and the galaxies are the Earth’s atmosphere and quakes…
The astronomers who didn’t stop for a missile strike (or a flat battery)
By Oli Usher, on 18 September 2015
This remarkable document comes from the observing log of the University of London Observatory. The observatory is now a UCL teaching facility and part of the Department of Physics & Astronomy, but at the time was an important research facility.
It reveals the effect of the Nazi bombing of London during the second world war.
UCL’s Bloomsbury campus was severely damaged in raids early in the war, with the Library’s dome destroyed and the historic buildings around the main quadrangle all gutted by fire.
Relentless bombing from aircraft early in the war, then by V1 and V2 missiles later in the war brought the conflict close to Londoners, and the unperturbed tone of the log makes clear how commonplace it had become – even in the quiet suburban environment of Mill Hill, where the observatory is located.
The log reads:
1944 August 3
Opened 21h. Sky clear.
Found red light on spectrograph not workingm, owing to plug having been left in and battery run down. Set on γ Cas with the help of dark room red light.
I started exposure at 21h 44m GMT.
Flying bomb exploded very close and shifted star in declination out of the field.
Star recovered and exposure restarted at 21h 47m GMT.
Just after starting the second time, a second flying bomb exploded. This was more distant and though it shifted image from the [spectrograph] slit, star did not go out of field and was quickly recovered.
Exposure ended 22h 07m GMT.
Exposure time = 20m.
Plate developed.
Closed 22h ½.
EMP CCLG
The event was a V1 flying bomb strike on nearby Hendon, which killed eight and destroyed 193 houses.
The signatures on the end of the document are of historical note too – alongside CCL Gregory, the director of the observatory, was EM Peachey. She is better known today under her married name of Margaret Burbidge. At the time, she was a young astronomer who had just passed her PhD, but she went on to become one of the preeminent astrophysicists of the twentieth century.
The observatory will be holding a one-day astronomy event in the Quad on 2 October, and an exhibition of astronomical images produced by UCL staff and students using its telescopes will be held along the walls of the North Cloisters throughout the term.
Why chemistry labs need expert glassblowers
By Oli Usher, on 11 September 2015
Chemistry labs need skilled glassblowers to produce some of the intricate, bespoke glassware for some of their experiments. This item, produced by one of UCL’s glassblowers, is designed to be placed on a heat source so it can distill liquids and deposit them in ampoules along its long stem.
The flask’s design includes bell-shaped edges inside to encourage the liquid to evaporate evenly. The gas then cools and condenses inside the long stem, collecting at the end. When enough has collected there, the end of the stem can be melted off, sealing the liquid safely inside.
Time turns backwards at the Spark Festival
By zcahe91, on 28 August 2015
While real time travel may be theoretically possible, it almost certainly won’t happen in our lifetimes. However, at the Spark Festival this weekend, the Chalkdust team will have a very surprising demonstration where we can turn time backwards.
For a sneak preview, watch this video of a Taylor-Couette cell (sound not necessary):
Surprised? I certainly was when I saw it for the first time.
For those who couldn’t watch the video: at first, blobs of coloured syrup are dropped into some clear syrup. Then the colours are mixed up by turning a tube inside the cylinder, until they look all brown. However, the tube is then turned back the other way, and the coloured liquid completely unmixes, ending up in the same blobs as they started. It looks exactly like the video has played backwards. It’s called reversible flow, and we’re very excited to have our own Taylor-Couette cell at the festival, where people of all ages can have a go at turning back time.
 Now you might be thinking – what’s that got to do with maths?
Now you might be thinking – what’s that got to do with maths?
A balanced view of radon
By Oli Usher, on 27 August 2015
Thin, fragile, light, and barely visible against the padding that keeps it intact, this object is nevertheless reflects an important period in the history of chemistry – a period in which UCL led the world.
Around the turn of the 20th century, UCL’s chemistry labs saw most of the key discoveries related to the noble gases. William Ramsay isolated helium and argon here in 1895, and went on to discover krypton, neon and xenon in 1898. Over the next few years, experiments at UCL proved that radon, discovered by Friedrich Dorn in Germany, also belonged to this group. (Ramsay won UCL’s first Nobel Prize for his work on these gases.)
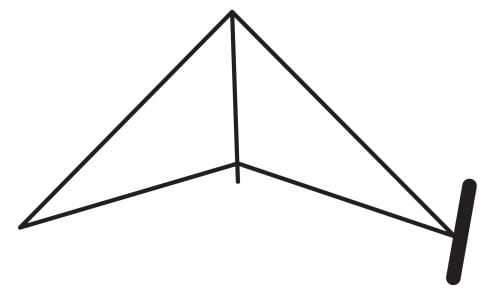
The delicate quartz balance in the image above is one of the most important pieces of apparatus used in this research on radon. It was used by UCL chemist Robert Whytlaw-Gray to weigh a sample of radon and determine its density for the first time. A schematic of the balance is shown below:
The balance held a tiny sample of radon in the chamber on its right, and was balanced inside a vacuum flask with a weight attached to the balance’s other side. Despite the tiny quantity of gas involved, this was enough to determine radon’s properties.
It is held by UCL’s chemistry collections, along with other artifacts relating to this period in UCL’s history, including William Ramsay’s Nobel Prize medal and citation.
Cantilevers for diagnosing disease
By Oli Usher, on 21 August 2015
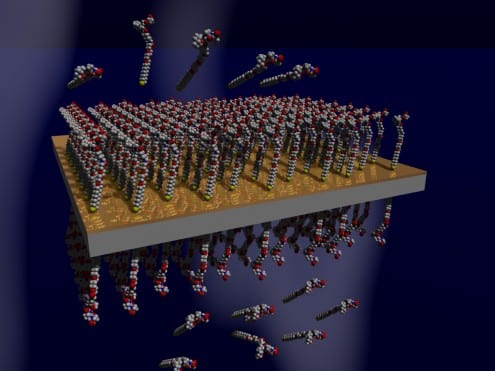
 This tiny device, developed by researchers at the London Centre for Nanotechnology, could soon help carry out difficult medical diagnoses.
This tiny device, developed by researchers at the London Centre for Nanotechnology, could soon help carry out difficult medical diagnoses.
The tiny rods, or ‘cantilevers’, are coated with molecules similar to those in our cells, which have been made sensitive to various diseases. In their work, the team have successfully made coatings which react with molecules that are part of HIV, the antibiotic Vancomycin, and blood anti-clotting factors (for haemophilia).
When in the presence of one of these molecules, the cantilever bends (as seen above), revealing the diagnosis.
 Close
Close