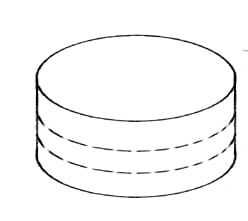
Amidst the indescribable stress that is writing up my PhD, there is a massive silver lining. I’m currently writing this from 2,800m (that’s about 9,200 feet), half way up the Mauna Kea volcano in Hawaii. I say volcano, it’s not actually erupted for several thousand years and (the reason that I’m here) it has billions of pounds worth of massive telescopes on top of it.

The peak of Mauna Kea, with Subaru, Keck 1, Keck 2 and NASA IRTF telescopes. Photo: Alan L (CC BY)
During the second year of my PhD, my supervisors and I, whilst looking at some data everyone had assumed was assumed was empty, discovered the first molecule containing a noble gas in it in space. Those of you who know anything about Chemistry will know this is really weird. Noble gasses are so named because they’re noble: they don’t mix with the other elements.
However, in the remnants of a star that exploded around 1000 years ago, the conditions for it to actually do so happened. This is a massive deal and needs following up quickly – which as well as taking over a substantial chunk of my time over the past year has now brought me to Hawaii.

The Crab Nebula – where UCL researchers discovered argon hydride molecules. Photo credit: NASA/ESA/Hester/Loll/Barlow
Last night was my first trip up to the summit, where I spend several hours at NASA’s Infrared Telescope Facility. It’s an odd experience being that high up. Everything needs to be a little bit slower. There are perpetual reminders that you are somewhere not normal. From the warning signs to the bottles of oxygen placed liberally around the control room.
People don’t function so well that high up.
Our trip was mostly to acclimatise to the 4,200m altitude and get used to the instruments we will be using. This is a good thing, because while Mauna Kea has 350 clear nights a year, last night was not one of them.
Last night there was a storm. The drive up to the summit was a pretty hairy experience with squalls of wind and rain. Thankfully it wasn’t me doing the driving.
The only work that needed doing last night was calibration set up, for which we didn’t need to be able to actually see stars. Just as well, as there’s no way that we could have.
This weather system should have passed by tomorrow so we’ll be free to do science.

NASA Infrared Telescope Facility. Photo: Afshin Darian (CC BY)
* * *
Night 2
Tonight’s drive up was much clearer. As well as stars we could see the top of a thunder storm out over the pacific and the orange glow from a neighbouring volcano (a nice reminder that although it hasn’t erupted for several thousand years, Mauna Kea is not actually extinct).
Clearer… until we got to the summit.

Inside the NASA Infrared Telescope Facility. Photo: Patrick Owen
Having prepared and calibrated everything and chosen our first standard star to use as a check for everything, the Telescope Operator said “no”.
Apparently it’s 100% humidity outside and it is lovely and misty.
We had a slight tease at about midnight: we got as far as opening the telescope dome and finding our standard star. Alas, just as we started taking actual measurements the humidity shot back up and we had to close the dome.
Night 3
There’s a massive difference when we get up to the summit tonight! Stars! I can see stars, not particularly brightly, which is mostly to do with the lack of oxygen at this altitude meaning my eyes aren’t working as well as they should, but stars!

NASA Infrared Telescope Facility at night. Photo: NASA
If I can see stars, the telescope can see stars. After some changing of instruments and refilling of coolants (no mean feat at that altitude) we were finally ready to get started. We found and observed out standard star without much of an issue.
Then we started looking for the little “knots” of gas we are observing in the Crab Nebula. This took us a while longer than planned, but we got there and all lined up on the instrument so we could get the data we need. Nothing. Tried again. Nothing. By this time it was also about 3am, the combination of the time and the lack of oxygen made this all rather difficult to cope with.

The Crab Nebula is full of knots and filaments of gas. Photo: NASA/ESA/Hester/Loll/Barlow
We set the telescope to run for a two hour run to see if we could get anything at all. Other than some cosmic rays (really not what we’re looking for at all) we got… nothing. Frustration and worry about our calculations and whether what we were doing was right ensued. I paced. Lots. As the sun came up I went outside to get some fresh air (and see the telescopes, I’d only been up here in the dark until now), before heading down to the base camp for some fried food and sleep.

The telescope at dawn, with crescent moon. Photo: Patrick Owen
Night 4
I woke up this “morning” to an email telling me that there had been something wrong with the instrument. Good news as it means we’re probably going to get some good data this evening. Bad news because it was a really simple fix that had we known about it would have allowed us to get some good data last night too.
Ah well, onwards and upwards. After another slog to get set up and find a new brighter standard star and things, we got observing.
Final night lucky, at about half past three, we finally realised we’d found what we were looking for! Massive amounts of relief all round, we still had to finish the run and get as much data as we could before the sun came up, but we got some.
It’ll take several weeks of processing the data followed by several more weeks of analysis before we know exactly what we have.
That can wait until after I’ve finished writing my thesis.

Sunset from base camp. Photo: Patrick Owen
Patrick Owen is a PhD student in UCL Physics & Astronomy, and has recently returned from observing in Hawaii

 Close
Close









 Of course, the secret is that the fate of our 100 micro-people is sealed: the urn will empty into the bath at the end of the show. Because it turns out social media chatter doesn’t change our fate – we need to act here in the physical, real world. The one in which the water is wet, the ice is melting, and the little people are struggling to stay dry.
Of course, the secret is that the fate of our 100 micro-people is sealed: the urn will empty into the bath at the end of the show. Because it turns out social media chatter doesn’t change our fate – we need to act here in the physical, real world. The one in which the water is wet, the ice is melting, and the little people are struggling to stay dry. That’s the reason the drowning is staged in a bath, with a hot-water urn above it, and tweets scrolling on a screen above that. All these activites – heating and processing water, and our endless online activity – they have a carbon footprint. They are all contributing to climate change.
That’s the reason the drowning is staged in a bath, with a hot-water urn above it, and tweets scrolling on a screen above that. All these activites – heating and processing water, and our endless online activity – they have a carbon footprint. They are all contributing to climate change.