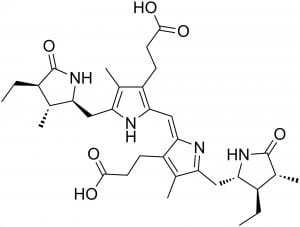
When the Pigment Timeline Project contacted him, former Grant Museum curator Mark Carnall nominated a list of pigments encountered in the Grant Museum (some of which may feature in later blogs). These include alizarin used to stain skeletons and orpiment used in arsenic soaps to preserve taxidermy specimens. However it is sepia brown that caught our attention. And we are not the first to be fascinated by this pigment.
“I was much interested, on several occasions, by watching the habits of an Octopus or cuttle-fish. Although common in the pools of water left by the retiring tide, these animals were not easily caught. They darted tail first, with the rapidity of an arrow, from one side of the pool to the other, at the same instant discolouring the water with a dark chestnut-brown ink.”—Charles Darwin, The Voyage of the Beagle (1839)
I expect taunting cephalopods whilst messing about in rock pools was as good as anything for passing the time on such a long sea voyage as Darwin endured on the Beagle. But somehow he managed to distract himself from this activity and focus his interests elsewhere. Nevertheless, the ‘chestnut-brown ink’ he noticed has long had a place in our societies. Everyone has heard of sepia. It’s a well known colour name, a translucent brown ink, which immediately evokes Victorian photographs for many people. But there is much more to this than just a colour name, indeed there is a story which goes back millions of years. The main modern source of sepia is the cuttlefish, Sepia officinalis, from which the ink sacs are extracted and are used for applications including medicines, as a kitchen ingredient and of course as an artists’ pigment. The latter two functions of this fluid have been combined by our colleague, printmaker Eleanor Morgan who used raw cuttlefish ink in her interpretation of the Japanese art of Gyotaku, producing prints of fish and other edible sea creatures. Once the print is made, you can eat the fish, ready basted in its sepia sauce.

Coleoid cephalopods for sale at Tavira fish market in Portugal. Octopus on the left and cuttlefish or ‘chocos’ on the right.

Dean Veall of UCL’s Grant Museum makes a Gyotaku print of an octopus using sepia ink at a workshop organised by Eleanor Morgan.
Ink is not restricted to cuttlefish. It is produced by all the coleoid cephalopods, i.e. squid, cuttlefish and octopus, who use it as a defence mechanism; a smoke-screen to confuse predators. Some deep water squids are even known to produce bioluminescent ink (Bush & Robison, 2007). The chemistry and biosynthesis of ink in cephalopods is complex and even to this day not well understood. It is a form of the common biological pigment melanin. Coleoid cephalopods produce the variety eumelanin which chemically and structurally is a complex polymer of 5,6-dihydroxyindole and 5,6-dihodroxyindole-2-carboxylic acid (Derby, 2014). Eumelanin is, as we all know, a dark brown, kind of ‘sepia’ colour … Melanins are also employed by cuttlefish and their relatives to change their skin colour by way of camouflage.
I visited the Grant Museum and met with Curator, Paolo Viscardi, to learn more about cephalopod ink in evolution. There is a large collection of cephalopods on display, preserved in glass jars, including several varieties of recent coleoids. However, without resorting to major dissection, the ink sacs were not visible. Nevertheless, these were rather beautiful objects.

Whilst looking at the samples of preserved cuttlefish in the display cases, Paolo asked me (leadingly, it transpires) if I was aware that the ink sacs developed at the embryonic stage in cepaholopods? I was not. He turned around and pointed out a microscope slide in the Museum’s Micrarium, with a perfect embryo of a Loligo sp. squid, that just happened to be right there! Stained pink with alizarin, three black patches are clearly visible in the tiny specimen, two of these are its eyes and in the centre of its body, very clearly visible, is the ink sac and duct, less than a millimeter in length.

Loligo sp. embryo – photo by Paolo Viscardi
The Grant Museum holds four of specimens of cephalopod fossils with excellent preservation of soft parts, including ink sacs. Surprisingly, sepia fossilises well. Glass et al. (2012) have studied fossilised ink sacs preserved in the Mid Jurassic Oxford Clay and Lower Jurassic Blue Lias Formations. They found that eumelanin was well preserved in these fossils and showed many similarities morphologically and chemically to that found in modern Sepia officinalis.
Paolo brought out the four fossilised cephalopods. One is on display in the Museum, the others we looked at in the stores. I have seen most places in UCL but this is the first time I have visited the Grant Museum’s dry stores. Whilst Paolo was locating the specimens, I gazed around at large cardboard boxes stacked on shelves, with marker pen labels like ‘disarticulated crocodile’ or ‘haddock x 27’.
The samples appeared and were splendid. They were obviously collected and prepared in the 19th Century and unfortunately coated in a good, thick layer of yellowing varnish, this has preserved them (though not a recommended conservation method today). Here they are:
This specimen (R132) shows exceptional preservation of the ink sac and duct in the cuttlefish Geoteuthis brevipi. The ink is produced in the bulbous gland and ejected through the duct, which connects with the anus of the cephalopod. You can work the rest out.

Belemnosepia sp. from the Oxford Clay at Christian Malford (R133). The ink sac is undamaged and appears as a bulbous protrusion on the surface.


Belemnites sp. (R28) with parts named. The remains of the ink sac are small and poorly preserved.

It is very likely that all of the three samples shown above were collected from a lagerstätte in the Oxford Clay near the village of Christian Malford in Wiltshire, which is justly famous for its well-preserved cephalopod fossils, including ammonites, belemnites and squids as well as fish and crustaceans. Indeed some of the samples analysed by Glass et al. (2012), came from this locality.
My favourite specimen in the Grant Museum came from Lyme Regis, a fragment of what would once have been a large cuttlefish with a huge ink sac, almost 12 cm long and also evidence of the cuttlebone (R110). The latter would probably have been aragonite, but the surface texture has preserved well and will be familiar to anyone who has ever owned a budgerigar. This was collected from ‘Lime Regis’ [sic], and is labelled Loligosepia Geoteuthis. Confusion may reign here, but these are two genus names and are synonymous, Loligosepia being the approved since 1966 (see Donovan & von Boletzky, 2014). However, like most of these things, most scientists ignore them and carry on naming things what they always used to name them. This could be the species Loligosepia bucklandi, originally discovered by #trowelblazer Mary Anning and named after protogeologist William Buckland.

Sepia-based artists’ materials are prepared from ink sacs extracted from modern cuttlefish. Terry (1893) writes in his ‘practical book for practical men’; ‘When the creatures are captured, their glands are carefully extracted and sun-dried to solidify the contents. In this state ink bags are sent into commerce. The colourman subjects the sacs to boiling in a solution of soda or potash, whereby the colour is dissolved out of the receptacle, and being filtered clear of all the animal tissue, is next precipitated by the addition of acid, collected on a filter, washed and dried.’ Sepia is usually mixed with the standard water colour medium gum Arabic. It was probably introduced as an artists’ pigment in the early nineteenth century (Eastaugh et al., 2008) and is still sold today by specialist pigment dealers.

A modern, dried ink sac from a cuttlefish ready to be prepared as an artist pigment.
Sepia, being dark brown, almost black, is not a rewarding pigment when viewed using polarising light microscopy and because of its complex organic chemistry it is not easy to identify in methods used routinely painting conservation scientists. Therefore it has not been identified in many works of art and is easily confused with other materials such as bistre and iron gall ink, however sepia ink is far more stable than these wood based inks (Reháková et al., 2015), if somewhat less vegetarian. Nevertheless it was probably frequently used for drawing, writing and as colour washes. It would be interesting to see how 160 million year old sepia would paint up …

Sepia pigment as a dispersion mount in plane polarised light; x 400 field of view is 1 mm (Eastaugh et al., 2008).
Find out more about The Grant Museum of Zoology
Coda: 11 October 2016
I have just been alerted to this marvellous blog post from OUMNH on the use of fossil sepia ink in the drawing of an Ichthyosaur skull by Elizabeth Philpott, 1833. So it has actually been used – and apparently very effectively as a pigment!

Coda: 24th October 2016
The fossil sepia as ink facts keep coming! This now from Henry Lee, Naturalist of the Brighton Aquarium in 1875 …

Billings, S, 2016, Tales from the Jurassic Coast., Oxford Museum of Natural History Blog
Bush, S.L. & Robison, B.H., 2007, Ink utilization by mesopelagic squid., Marine Biology, 152, 485–494.
Derby, C. D., 2014, Cephalopod Ink: Production, Chemistry, Functions and Applications., Marine Drugs, 12, 2700-2730.
Donovan, D. T. & von Boletzky, S., 2014, Loligosepia (Cephalopoda: Coleoidea) from the Lower Jurassic of the Dorset coast, England., N. Jb. Geol. Paläont. Abh. 273/1, 45–63.
Eastaugh, N., Walsh, V., Chaplin, T., & Siddall, R., 2008, Pigment Compendium: A Dictionary and Optical Microscopy of Historic Pigments. (1st ed.). London: Butterworth-Heinemann., 343, 776-7.
Glass, K., Ito, S., Wilby, P. R., Sota T., Nakamura, A., Bowers, C. R., Vinther, J., Dutta, S., Summons, R., Briggs, D. E. G., Wakamatsu, K. & Simon, J. D., 2012, Direct chemical evidence for eumelanin pigment from the Jurassic period., PNAS, 109 (26), 10218–10223.
Lee. H., 1875, The Octopus or the ‘Devil-Fish’ of fiction and of fact., Chapman and Hall, London., 97-98.
Reháková, M., Ceppan, M., Vizárová, K., Peller, A., Stojkovičová, D., & Hricková, M., 2015, Study of stability of brown-gray inks on paper support., Heritage Science, 3 (8), 7 pp.
Terry, G., 1893, Pigments, paint & painting: a practical book for practical men. E. & F. N. Spon, London & New York.

 Close
Close



 Yellow: Sulphur Although Sulphur is not a pigment, I found some in a curio shop and was curious to see if it ground into a powder; would it work in the same way? So I intend to turn it into watercolour and test it out. Historically it has been used to bleach cloth, so it might do something similar when applied over the top of other watercolours. Sulphur occurs naturally as the element, often in volcanic areas, and as the extraction of pigments is very alchemical, I thought it was interesting to note that for centuries, along with mercury and salt, it was believed to be a component of all metals and formed the basis of alchemy, whereby one metal could be transmuted into another.
Yellow: Sulphur Although Sulphur is not a pigment, I found some in a curio shop and was curious to see if it ground into a powder; would it work in the same way? So I intend to turn it into watercolour and test it out. Historically it has been used to bleach cloth, so it might do something similar when applied over the top of other watercolours. Sulphur occurs naturally as the element, often in volcanic areas, and as the extraction of pigments is very alchemical, I thought it was interesting to note that for centuries, along with mercury and salt, it was believed to be a component of all metals and formed the basis of alchemy, whereby one metal could be transmuted into another.
 Blue: Azurite Azurite is a soft copper mineral, named for its beautiful “azure blue” colour. It has been ground and used as a pigment in blue paint as early as ancient Egypt, and through time, its become much more common. During the Middle Ages and Renaissance, it was the most important blue pigment used in Europe, and through the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France, where much of the pigment was mined. Here I have mullered the Azurite into glaze.
Blue: Azurite Azurite is a soft copper mineral, named for its beautiful “azure blue” colour. It has been ground and used as a pigment in blue paint as early as ancient Egypt, and through time, its become much more common. During the Middle Ages and Renaissance, it was the most important blue pigment used in Europe, and through the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France, where much of the pigment was mined. Here I have mullered the Azurite into glaze. Indigo: Mussel Shell Blue from St Ives Since landing in St Ives I have been going down to the foreshore every morning to collect mussel shells as I wanted to create a blue pigment to represent the sea, however after being ground the mussels create a light indigo colour that I love! Historically painters used shells as paint pans, so I thought it very appropriate to make watercolour paint with the mussel pigment and use one of the mussel shells as the pan for the paint.
Indigo: Mussel Shell Blue from St Ives Since landing in St Ives I have been going down to the foreshore every morning to collect mussel shells as I wanted to create a blue pigment to represent the sea, however after being ground the mussels create a light indigo colour that I love! Historically painters used shells as paint pans, so I thought it very appropriate to make watercolour paint with the mussel pigment and use one of the mussel shells as the pan for the paint. Violet: Cochineal Cochineal is a bright scarlet insect lake pigment that has been used for centuries to dye textiles, drugs, food and cosmetics. A lake pigment is a pigment made by precipitating a dye with a mordant. Unlike mineral pigments, lake pigments are organic.Cochineal is the result of harvesting the female cochineal parasitic insect that live on the cacti native to Mexico, Central and South America. Using soda ash and alum, I extracted the pigment from the insects and added honey and gum arabic to make watercolour.
Violet: Cochineal Cochineal is a bright scarlet insect lake pigment that has been used for centuries to dye textiles, drugs, food and cosmetics. A lake pigment is a pigment made by precipitating a dye with a mordant. Unlike mineral pigments, lake pigments are organic.Cochineal is the result of harvesting the female cochineal parasitic insect that live on the cacti native to Mexico, Central and South America. Using soda ash and alum, I extracted the pigment from the insects and added honey and gum arabic to make watercolour.











































































 The universally accepted method of removing the lamp black from the surface seems to be by scraping it off with a feather. In addition to Li Qiaoping’s descriptions, Jacobean gentleman Henry Peacham wrote in his treatise ‘
The universally accepted method of removing the lamp black from the surface seems to be by scraping it off with a feather. In addition to Li Qiaoping’s descriptions, Jacobean gentleman Henry Peacham wrote in his treatise ‘