A Colour A Day: Week 47
By Ruth Siddall, on 14 February 2021
A Colour A Day: Week 47 8th – 14th February
Jo Volley writes…
This week’s colours accompany this beautiful poem by artist and poet Sharon Morris.
The purpose of blue
But it’s the colours I miss, don’t you see?
the lapis sky and fair cerulean blue
of ocean, the precise shivering hue
of your laugh on a bright day, so clear.
Whatever the light, lavender appears
to shave blue from grey, the way I knew you.
I’m dead-heading the daisy – though it’s futile –
sweeping leaves and weeding ‘volunteers’.
My eyes close – the way whales slip from view
between the waves – I have to let you go.
I still wear that specific shade of turquoise –
you looking out at the Pacific Ocean –
the way blue sky screens emptiness, its purpose
forgetting or holding on. Is this beauty?
The purpose of blue is from a set of sonnets, some of which were published in the anthology Tying the Song, Enitharmon Press, 2000. Sharon is also a Professor of Fine Art, Slade Deputy Director (Academic) and Head of the PhD Programme.
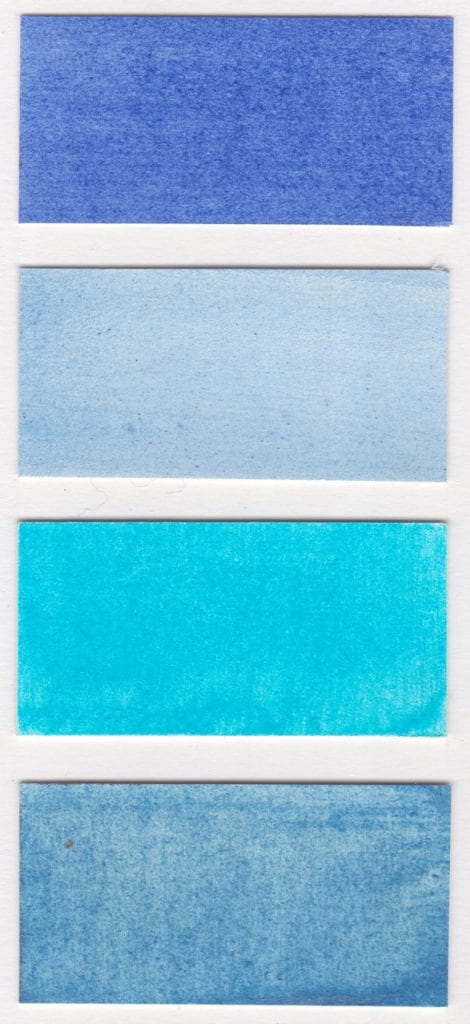
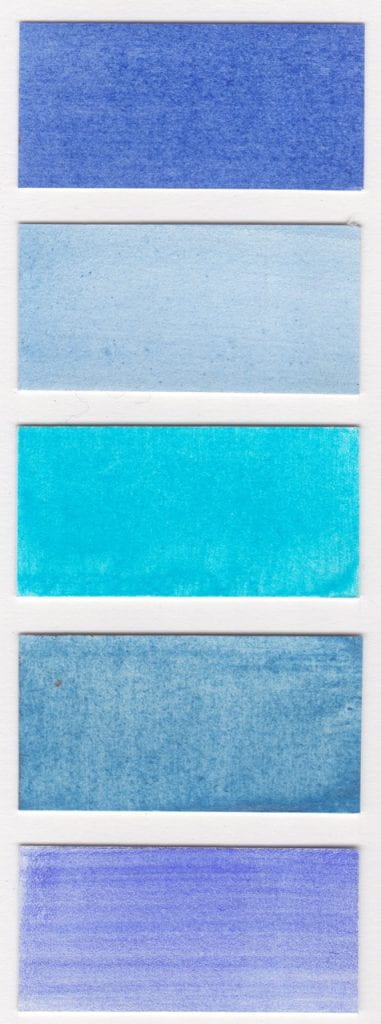
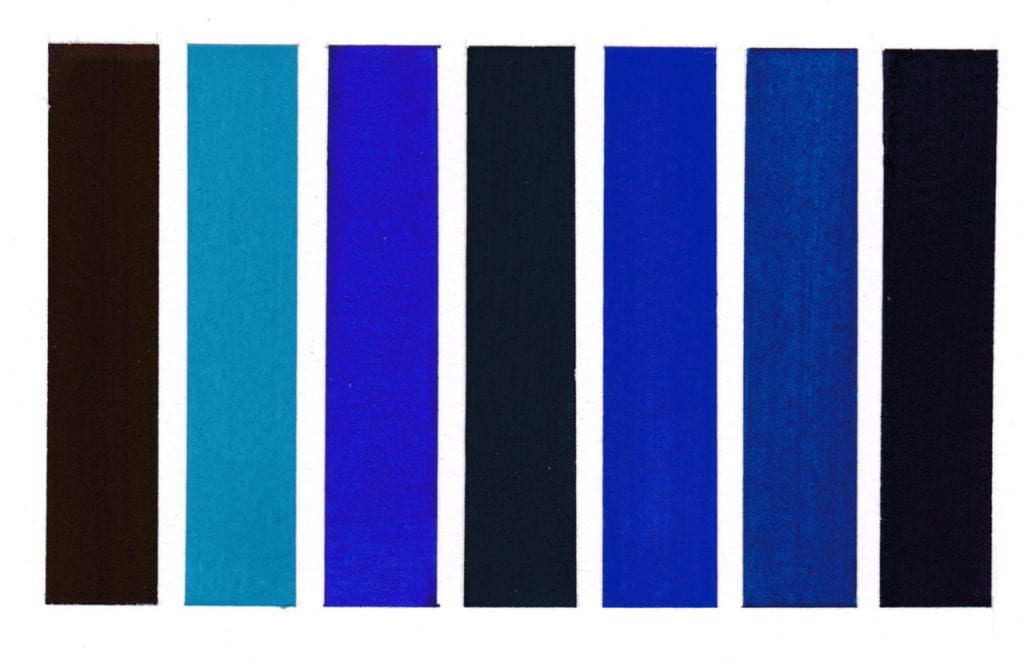
Colours read from left to right on W&N watercolour paper.
Methyl Violet pigment bound in gum Arabic
Cerulean Blue pigment bound in gum Arabic
Dumont’s Blue W&N watercolour
Vivianite pigment bound in gum Arabic
Oregon Blue (Yin mIn Blue), Derivan, Matisse Range*
Monastral Blue pigment bound in gum Arabic
Bronze Blue pigment bound in gum Arabic
 *I first became aware of Yin MIn Blue in the summer of 2016 and wrote to the manufacturers requesting a sample for the Slade Material Research Project Pigment Collection but without luck. I then discovered a paint manufacturer in Australia, Derivan, were advertising it in their Matisse range as Oregon Blue and wrote a similar email asking for a donation. This is response from Steven Patterson, Derivan’s Chief Executive Officer that summer.
*I first became aware of Yin MIn Blue in the summer of 2016 and wrote to the manufacturers requesting a sample for the Slade Material Research Project Pigment Collection but without luck. I then discovered a paint manufacturer in Australia, Derivan, were advertising it in their Matisse range as Oregon Blue and wrote a similar email asking for a donation. This is response from Steven Patterson, Derivan’s Chief Executive Officer that summer.
‘Thank you for your email – I would be happy to send you a sample of the paint we have made with the pigment, however we do not have any dry pigment left!!! we have used it all!!! – yet if you are happy with the paint please let me know the best address to send it to.’
I accepted his kind offer and very excited to receive a few weeks later two tubes plus some lovely colours from their Natural Pigments of Australia range which have been featured in previous weeks A Colour A Day.
A conversation in the Housman bar over the newly acquired blue with Ruth Siddall and David Dobson got David thinking about inventing his own new blue – more on that another time. At a later date Steven Patterson very generously sent a sample of the pigment, now part of the collection, and featured in an exhibition in the Material Museum during Colour & Poetry: A Symposium 2019.
 Close
Close