Neurulation biomechanics lab
By Gabriel Galea, on 26 March 2024
The Neurulation Biomechanics (Galea) Lab aims to improve prediction, prevention and patient outcomes for those affected by neural tube defects such as spina bifida. Our research relies on three key experimental systems: animal models of human congenital malformations, patient-derived tissues, and human induced pluripotent stem cells. We strive to bridge the gap in our understanding between genetic/molecular mechanisms and their tissue-level consequences which underlie congenital malformations. By combining different experimental systems, our research ranges from fundamental studies of key cellular behaviours to translational work aiming to improve outcomes for individuals who have spina bifida.
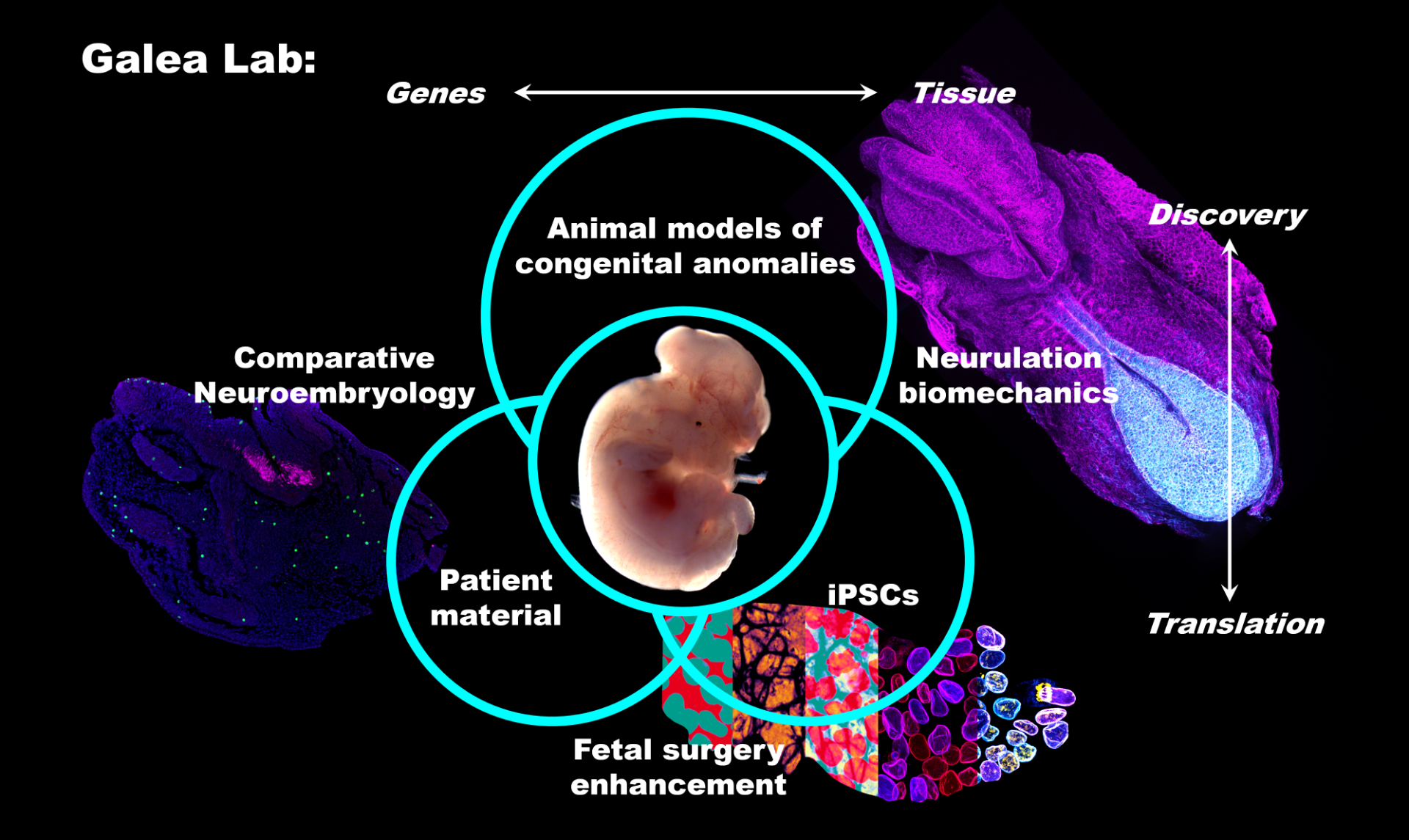
New mouse model of Terminal Myelocystocele
By Gabriel Galea, on 26 March 2024
Our recently-published article shows that regional deletion of Fgfr1 in the embryonic trunk produces localised spinal mis-patterning and a terminal myelocystocele-like phenotype in mice. The image below shows a normal mouse fetus (left) and one with a sac-like protrusion at the bottom of the spinal cord (right) which resembles a rare human malformation called Terminal Myelocystocele. Our paper shows that this malformation does not arise because of failed neural tube closure, like more common types of spina bifida do, but happens are the neural tube is fully closed.
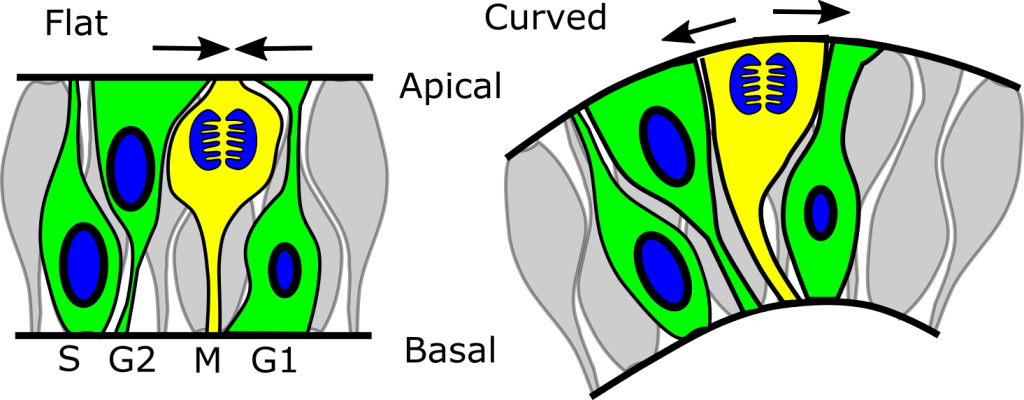
Neuroepithelial synchronisation of apical constriction and mitosis
By Gabriel Galea, on 12 December 2022
Our latest paper shows that neuroepithelial cells undergo high-amplitude apical constriction synchronised with cell cycle progression but the timing of their constriction if influenced by tissue geometry. We observe this in mouse and chick embryos, as well as in human iPSC-derived neuroepithelial cells cultured on either flat or curved surfaces. We find this human cell model particularly tractable, except that it requires daily medium changes to keep the cells happy!
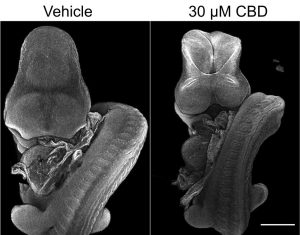
Cannabidiol impairs neural tube closure in mouse whole embryo culture
By Gabriel Galea, on 13 April 2022
In our latest paper, we find that exposing mouse embryos to cannabidiol (CBD) in whole embryo culture diminishes their ability to close the neural tube. This happens at concentrations which do not impair the development of other embryonic structures such as somites and does not change cell proliferation or apoptosis, suggesting a selective effect on the neural tube. It’s important not to over-interpret this paper: we exposed embryos to CBD directly not from their mother and we do not know what the mechanism behind the observed effect is. Congratulations to Yosuf who completed most of this work during his iBSC as part of his UCL medical degree.
You can access the paper here.
Measuring the mechanical forces generated by embryos
By Gabriel Galea, on 18 February 2022
Check out our latest pre-print in which we quantify the mechanical forces generated by the closing neural tube in chick embryos. it still feels like science fiction: massive congratulations to Eirini for completing this exciting work.
You can access the pre-print on Research Square, here.
How does the brain get covered with skin?
By Gabriel Galea, on 12 May 2021
Our latest paper combines physics, embryology and advanced microscopy to work out how the brain gets covered with skin. Read the original paper here, or just watch it happening below!
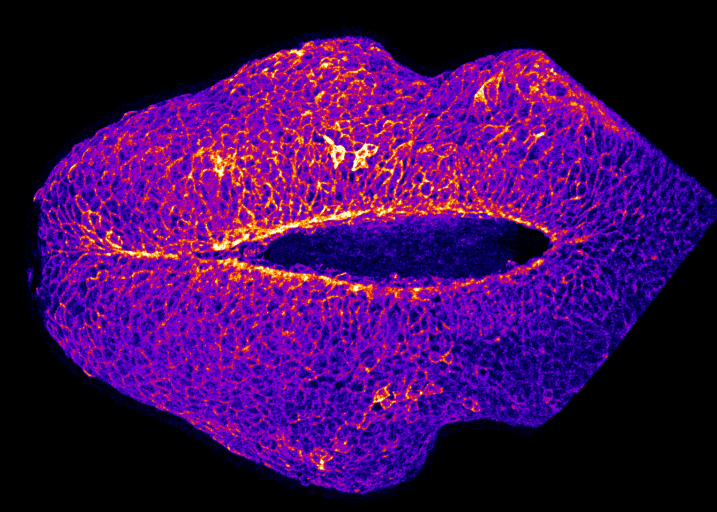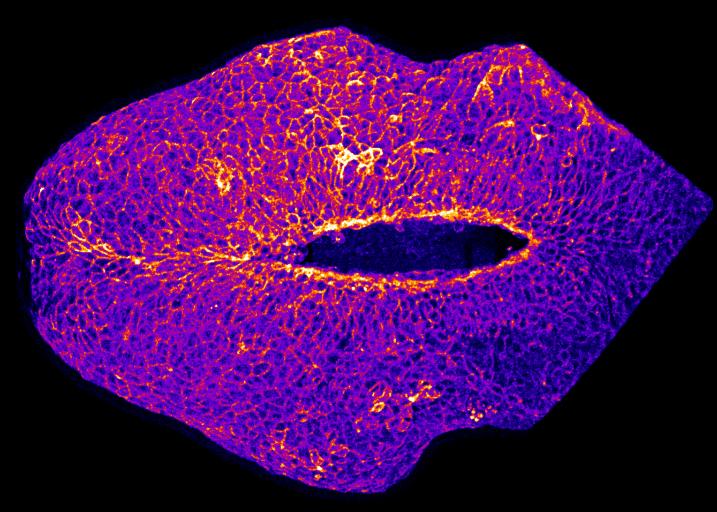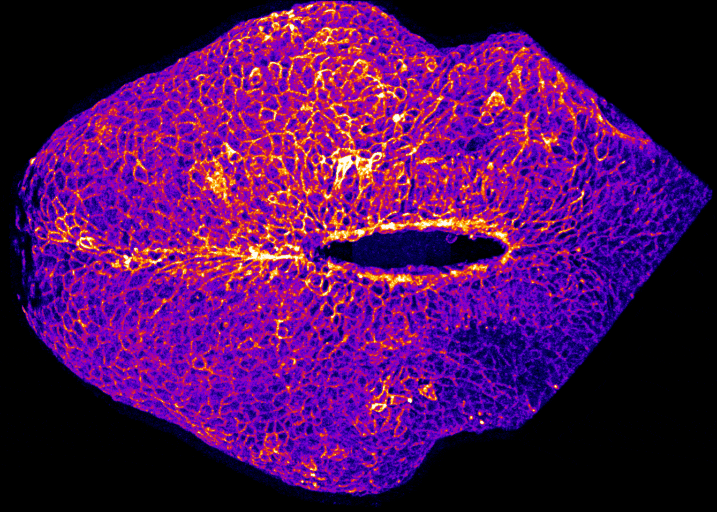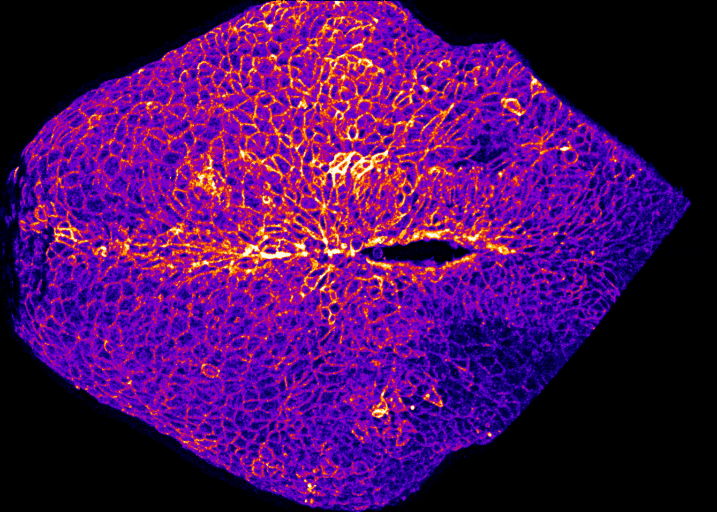
Live-imaging showing progression of hindbrain neuropore closure in a mouse embryo.
Uninherited mutations can cause spina bifida
By Gabriel Galea, on 19 February 2021
Our latest research, published in Nature Communications, reveals that spinal cord formation is exquisitely susceptible to mutations which happen during embryo development. We find that mutating one gene, called Vangl2, in just 16% of developing spinal cord cells is enough to cause spina bifida in mice. This is because each mutant cell interferes with the normal function of its neighbours. These mutations are not inherited from either parent, and would not be passed on to the individuals’ children.
We already knew that uninherited (“somatic”) mutations in genes which interact with Vangl2 can be found in 15% of individuals who have spina bifida (previous paper here), but we did not know if these rare mutations were enough to cause such a severe birth defect. We now need to improve diagnostic testing methodology in order to find these mutations without needing to cut out patient tissue.
You can read a lay interpretation of our study here.
Development journal cover figure
By Gabriel Galea, on 27 December 2020
We recently collaborated with the Sowden lab at the Institute of Child Health, providing imaging expertise to study eye development. One of the figures generated in the course of that research has been selected as the cover figure of the journal Development. This figure shows delicate structures in the developing human eye including the lens and retina. The developing eye shares many cellular similarities with the closing neural tube.

Making and shaping bone
By Gabriel Galea, on 14 December 2020
A few years ago we reported that gene mutations which predispose to spina bifida independently diminish bone mass in mice. Now, in collaboration with Prof Philippa Francis-West at King’s College, we’ve completed a wide-ranging invited review of the mechanisms by which bone is made and shaped in the embryo. With over 350 references read and cited, it really is a behemoth of a review!
In it we define critical cellular behaviours which shape skeletal elements during fetal development and raise questions which need to be addressed in future studies. Read all about it here.
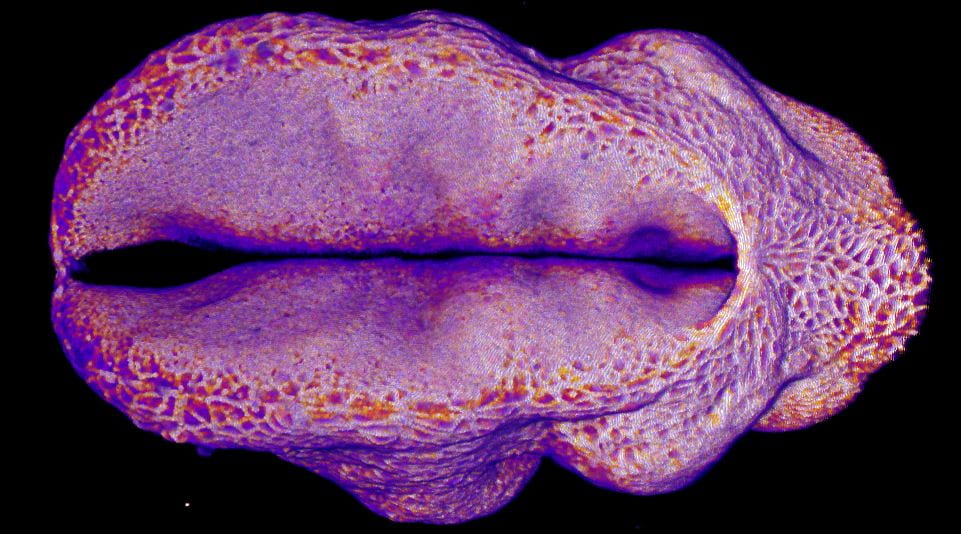
Hindbrain neuropore closure
By Gabriel Galea, on 3 November 2020
Here’s a link to our new pre-print showing that hindbrain neuropore tissue geometry determines asymmetric cell-mediated closure dynamics. The hindbrain neuropore is a tissue gap over the back of the head which needs to close in order to cover the developing brain with other other cell types. If that does not happen the embryo develops a fatal birth defect called exencephaly (also called anencephaly). Eirini’s work, shown in this pre-print, identifies two different behaviours by which cells around this gap generate mechanical forces needed to close it. Thanks to our collaborations with physicists at Carnegie Mellon, we were able to show that both these behaviours must happen at the same time to describe closure of this gap.
In the image below, the top of the head is on the left, the neck is on the right, and the massive hole between them is the hindbrain neuropore.
 Close
Close