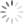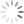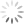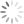Welcome to the Neurulation Biomechanics Lab!
We’re a growing group of scientists at the UCL Great Ormond Street Institute of Child Health studying how our brain and spinal cord form while we are tiny embryos.
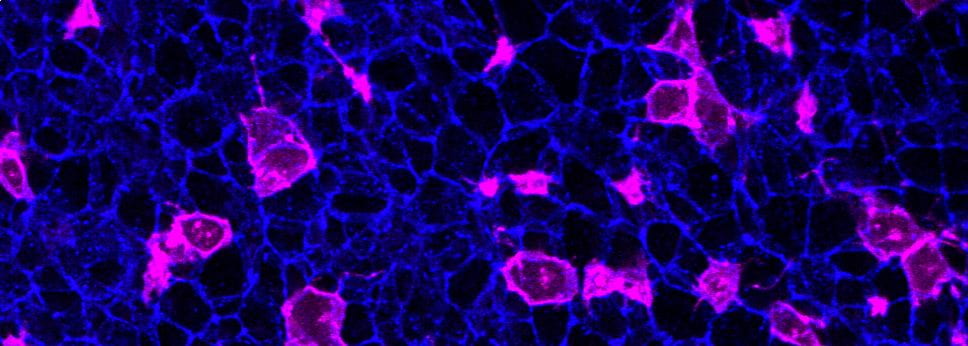
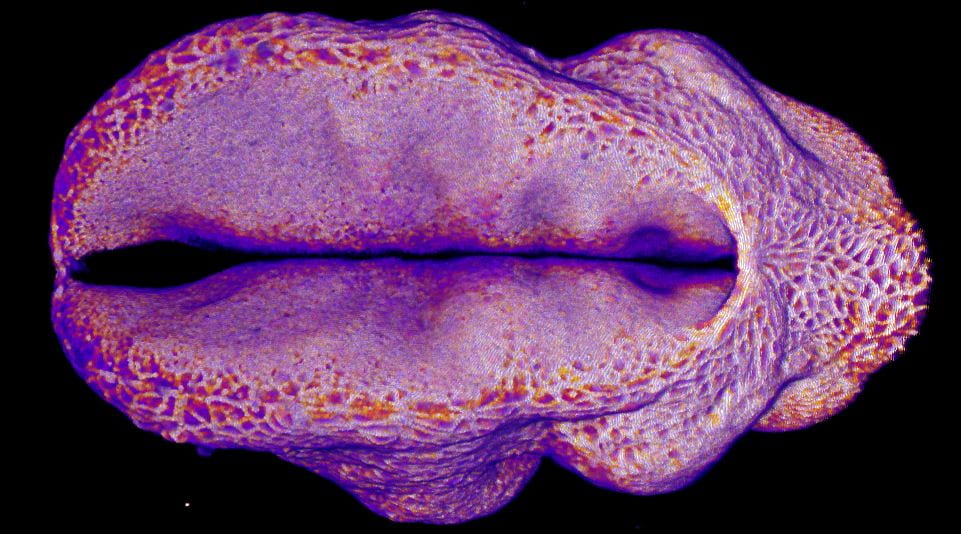
Specifically, we study how embryos change their shape to cover the brain and spinal cord with skin which protects them during development. If that doesn’t happen, the embryo develops a birth defect known as a Neural Tube Defect. Examples of Neural Tube Defects include Spina Bifida. We study this using genetic models and advanced microscopy, which allows us to watch the neural tube close in model species including mice and chicks.
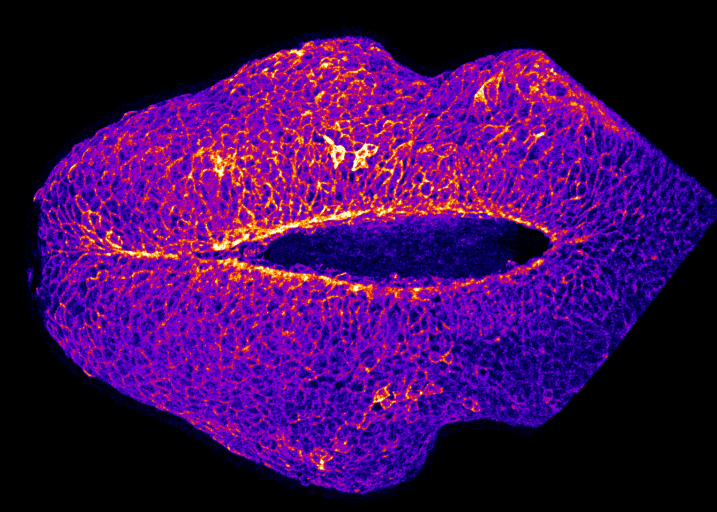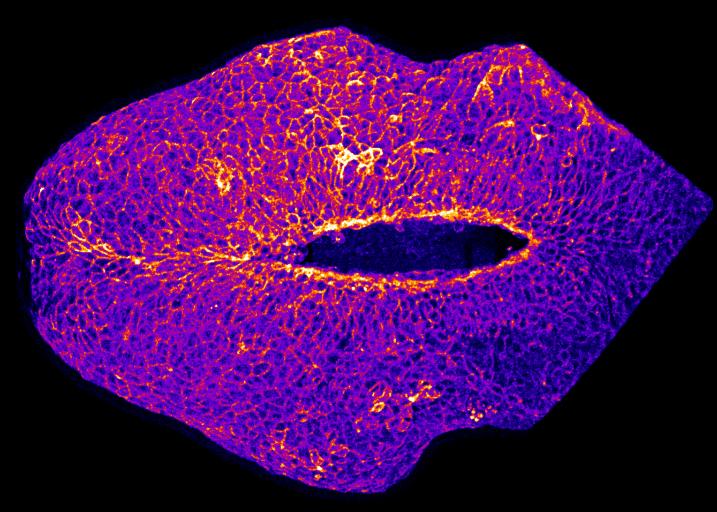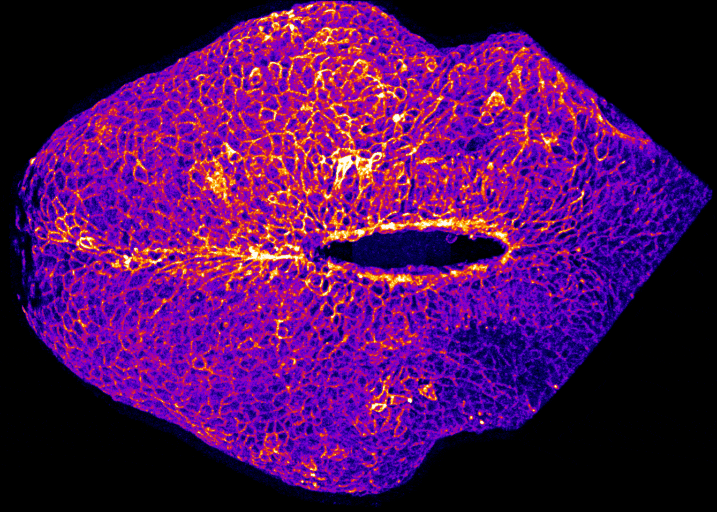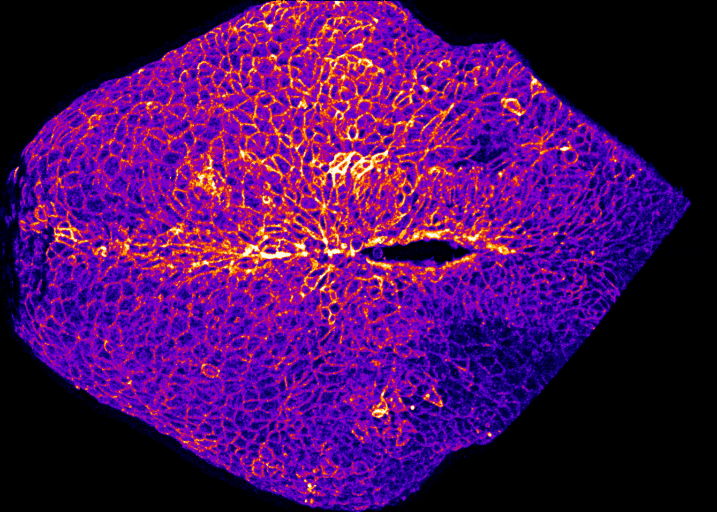
In the image on the right you can see the tail-end of a mouse embryo. Click on it to watch it move. The cyan region is the future skin, which you will see encircles a folding region of red cells. These red cells will form the future spine and you can see how, over around two hours of live-imaging, the embryo makes a lot of progress in covering it. Clearly, the embryo needs to change its shape quite a lot! What you’re seeing is the embryonic process of neurulation.
How do embryonic cells generate, withstand and transmit the mechanical forces required to change the shape of this tissue? This is what we mean by Biomechanics – the mechanics of biological systems.
Do abnormalities in these processes cause Neural Tube Defects? Can we apply what we learn to prevent, or at least explain the causation of, conditions like Spina Bifida? These are many of the questions our lab is interested in and we hope to share our findings here over years to come.
 Close
Close