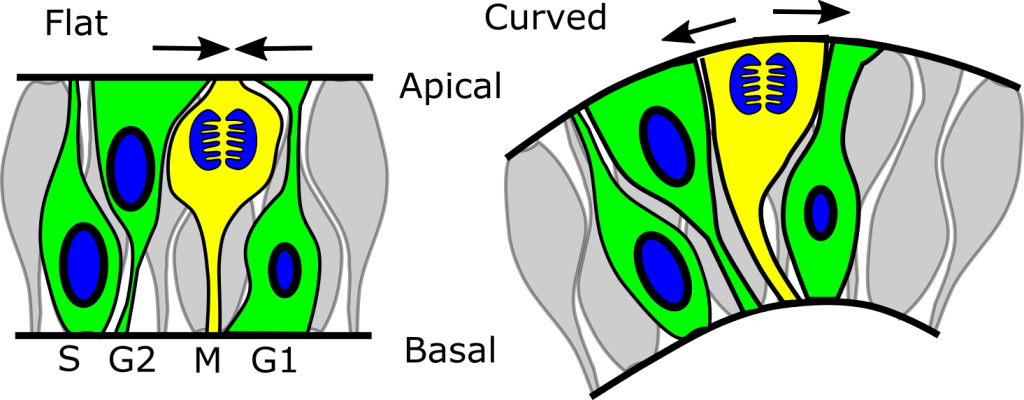
New mouse model of Terminal Myelocystocele
By Gabriel Galea, on 26 March 2024
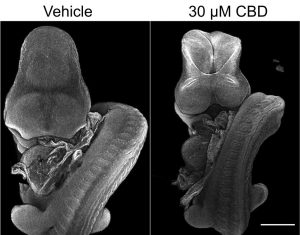
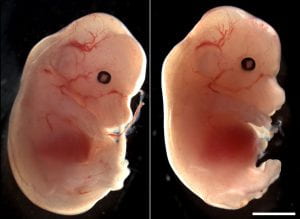
Our recently-published article shows that regional deletion of Fgfr1 in the embryonic trunk produces localised spinal mis-patterning and a terminal myelocystocele-like phenotype in mice. The image below shows a normal mouse fetus (left) and one with a sac-like protrusion at the bottom of the spinal cord (right) which resembles a rare human malformation called Terminal Myelocystocele. Our paper shows that this malformation does not arise because of failed neural tube closure, like more common types of spina bifida do, but happens are the neural tube is fully closed.
 Close
Close