In this Cell & Gene Therapy TIN interview as part of the Early Career Innovators series, recognising the amazing translational work being done by postdoc and non-tenured researchers within the UCL Therapeutic Innovation Networks (TINs), Dr Marion Mercier highlights her Cell & Gene Therapy TIN Pilot Data Fund awarded project, involving the validation of novel gene therapies for epilepsy.
What is the title of your project and what does it involve?
Human brain tissue is routinely excised during epilepsy surgery, and can, given the right conditions, be maintained alive in slice culture for extended periods of time. My project, entitled “Validating novel AAV gene therapies for epilepsy in human organotypic slices”, involves firstly to optimise human tissue slicing and culture protocols for the successful maintenance of this tissue, and secondly to establish efficient viral transfection methods in these human organotypic slices. The specific virus used encodes for a protein that suppresses neuronal excitability and as such is being developed as a gene therapy strategy for epilepsy. Thus, the project aims to establish a human tissue model in which to validate and screen this, and future, gene therapies for epilepsy developed within the DCEE.
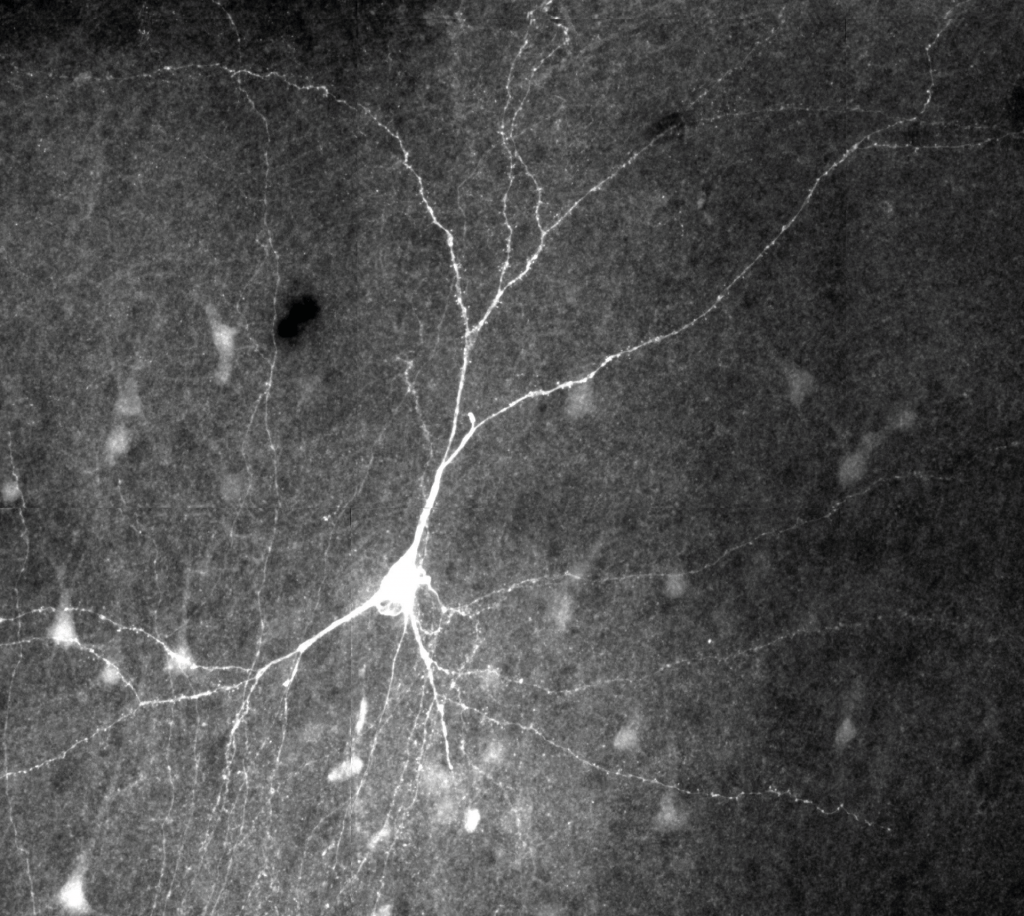
Filled and stained human pyramidal cell.
What is the motivation behind your project/therapeutic?
Epilepsy affects 1% of the global population, and 30% of patients are pharmaco-resistant, with significant associated morbidity. Several novel gene therapies for epilepsy have recently been identified and developed within the DCEE, and offer real hope for these patients. However, while results from animal models have been promising, understanding how these genetic manipulations, and the adeno-associated viral vectors (AAVs) used to deliver them, will behave in the human brain still poses a significant challenge. Furthermore, the irreversible nature of gene therapy makes transitioning from animal models to human patients particularly risky. By establishing human organotypic slices to extend the viability of excised human brain tissue, and thereby enabling transfection with AAVs (which take 2-3 weeks to express), I aim to develop a human neuronal tissue model in which to screen and validate these novel gene therapies for epilepsy and thereby help to bridge this important translational gap.
Can you highlight any challenges have you experienced as an early career researcher in the cell and gene therapy/translational research space?
Obtaining funding for your own independent ideas and research is particularly challenging as an early career researcher, and is often impossible without considerable preliminary data. This makes getting started on new projects, and gaining the independence necessary to progress on to more senior, permanent positions, especially difficult. Furthermore, as an early career researcher working at the intersect between clinical and more basic science, I have found the complex translational research pathway quite challenging to navigate.
What do you hope to achieve in the 6 months duration of your project?
I have two main objectives for the 6 months duration of the project. The first is to establish good quality human organotypic slices that are viable for up to 3 weeks, and the second is to develop effective viral transfection methods in these slices. I will be transfecting the tissue with AAV-hCaMKII-EKC-GFP, a virus that aims to increase expression of an enhanced K+ channel (EKC) in human excitatory neurons, and which has shown promise as a gene therapy strategy in animal models of epilepsy. Thus, while optimising protocols for viral transfection of human organotypic slices, I hope to also start to collect clinically-relevant data pertaining to the safety of the viral transfection and the selectivity of the expression. I am currently in the first phase of the project and have already improved the human tissue slicing protocol and started to optimise the slice culture methods.

Why did you want to apply to the Cell & Gene Therapy TIN Pilot Data Fund?
In order to start this project, all I required was two specialised pieces of equipment and a little extra funding for consumables. The Cell and Gene Therapy TIN Pilot Data Fund is ideally suited for this, and therefore provides the perfect stepping stone for getting started and obtaining quality preliminary data with which to then apply for further funding. Furthermore, it has enabled me to progress my research in a more translational direction, and to learn more about the translational pathway and all of the steps involved in getting a therapy from the lab to the clinic. This will not only be an invaluable help in establishing and advancing this current project, but also in informing my future research plans.
We are currently in the process of determining our funding availability for the Cell & Gene Therapy TIN for 2021. Please join the Cell & Gene Therapy TIN and sign up to the TIN newsletter to keep updated.
How did you find the process for the TIN Pilot Data Fund? What did you learn?
The application process was rewarding and a great learning experience. I attended the ACCELERATE coaching session on pitching projects, through which I learnt a great deal about how to communicate my ideas effectively, concisely and convincingly. Receiving this training prior to the interview made the final pitching exercise exciting rather than daunting and made it an overall positive experience through which I received a lot of constructive feedback. This has given me more confidence in my ideas and capabilities and pushed me to be more competitive and ambitious in driving my research forward.
About Dr Marion Mercier

Dr Marion Mercier a postdoctoral researcher in Prof. Dimitri Kullmann’s laboratory within the UCL Institute of Neurology’s Department of Clinical and Experimental Epilepsy (DCEE). After an undergraduate degree in Psychology at Reading University and a year as a technician working on drug discovery for epilepsy, Marion moved to Bristol to do her PhD in the laboratory of Prof. Graham Collingridge where she studied glutamate transmission and synaptic plasticity in the hippocampus.
Throughout her postdoctoral work, her research interests have evolved at the intersect between basic and clinical neuroscience, focusing specifically on interneuron plasticity and synaptic function in both physiological states and pathological conditions such as epilepsy. Recently, she has begun to study human cortical function in resected human brain tissue, and is interested in establishing human neuronal models from this tissue in order to validate the gene therapy strategies for epilepsy currently being developed within the DCEE.
 Close
Close








