Pigment Stories: Polly Bennett’s Pigment Rainbow
By Ruth Siddall, on 23 March 2020
Yesterday (22 March 2020) we launched the inaugural World Pigment Day. There was an huge amount of engagement on social media and particularly on Instagram. Over the next few days I will be sharing images and pigment stories from people who posted to celebrate World Pigment Day. First up is artist Polly Bennett, a resident of St Ives in Cornwall, who contributed a series of posts on the colours of the Rainbow. Over to Polly …
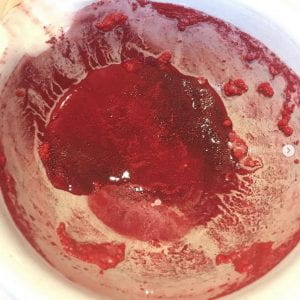
Red: Cinnabar Cinnabar is a toxic mercury sulfide mineral that has been used as a pigment for thousands of years due to its bright red colour. It is a pigment in its own right, however, it was also used to make the red pigments known as “vermilion” and “Chinese red”. Cinnabar is a hydrothermal mineral that is usually found in rocks surrounding recent volcanic activity but can also form near hot springs and fumaroles (an opening in or near a volcano). Because of cinnabar’s toxicity, it is a lot less commonly used nowadays.

Orange: Ochre Ochre is a family of earth pigments that includes yellow ochre, red ochre, purple ochre, sienna, and umber. It consists of varying amounts of iron oxide, clay and sand, and ranges in colour from yellow to deep orange or brown with an array of shades inbetween. I have found huge amounts of ochre earth in St Ives, where I am currently staying, and have been slowly but surely grinding and separating the ochre into different shades. The mineral goethite, an iron oxide hydroxide and the main constituent of most yellow ochres, is named after Johann Wolfgang von Goethe, the colour scientist whose death marks the date of World Pigment Day
 Yellow: Sulphur Although Sulphur is not a pigment, I found some in a curio shop and was curious to see if it ground into a powder; would it work in the same way? So I intend to turn it into watercolour and test it out. Historically it has been used to bleach cloth, so it might do something similar when applied over the top of other watercolours. Sulphur occurs naturally as the element, often in volcanic areas, and as the extraction of pigments is very alchemical, I thought it was interesting to note that for centuries, along with mercury and salt, it was believed to be a component of all metals and formed the basis of alchemy, whereby one metal could be transmuted into another.
Yellow: Sulphur Although Sulphur is not a pigment, I found some in a curio shop and was curious to see if it ground into a powder; would it work in the same way? So I intend to turn it into watercolour and test it out. Historically it has been used to bleach cloth, so it might do something similar when applied over the top of other watercolours. Sulphur occurs naturally as the element, often in volcanic areas, and as the extraction of pigments is very alchemical, I thought it was interesting to note that for centuries, along with mercury and salt, it was believed to be a component of all metals and formed the basis of alchemy, whereby one metal could be transmuted into another.

Green: Green Earth from St Ives Yesterday I was super excited to find a little green sparkly rock on the eroded foreshore. I set about grinding it down and managed to get two shades of green from it, the darker one I immediately made into watercolour.
 Blue: Azurite Azurite is a soft copper mineral, named for its beautiful “azure blue” colour. It has been ground and used as a pigment in blue paint as early as ancient Egypt, and through time, its become much more common. During the Middle Ages and Renaissance, it was the most important blue pigment used in Europe, and through the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France, where much of the pigment was mined. Here I have mullered the Azurite into glaze.
Blue: Azurite Azurite is a soft copper mineral, named for its beautiful “azure blue” colour. It has been ground and used as a pigment in blue paint as early as ancient Egypt, and through time, its become much more common. During the Middle Ages and Renaissance, it was the most important blue pigment used in Europe, and through the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France, where much of the pigment was mined. Here I have mullered the Azurite into glaze.
 Indigo: Mussel Shell Blue from St Ives Since landing in St Ives I have been going down to the foreshore every morning to collect mussel shells as I wanted to create a blue pigment to represent the sea, however after being ground the mussels create a light indigo colour that I love! Historically painters used shells as paint pans, so I thought it very appropriate to make watercolour paint with the mussel pigment and use one of the mussel shells as the pan for the paint.
Indigo: Mussel Shell Blue from St Ives Since landing in St Ives I have been going down to the foreshore every morning to collect mussel shells as I wanted to create a blue pigment to represent the sea, however after being ground the mussels create a light indigo colour that I love! Historically painters used shells as paint pans, so I thought it very appropriate to make watercolour paint with the mussel pigment and use one of the mussel shells as the pan for the paint.
 Violet: Cochineal Cochineal is a bright scarlet insect lake pigment that has been used for centuries to dye textiles, drugs, food and cosmetics. A lake pigment is a pigment made by precipitating a dye with a mordant. Unlike mineral pigments, lake pigments are organic.Cochineal is the result of harvesting the female cochineal parasitic insect that live on the cacti native to Mexico, Central and South America. Using soda ash and alum, I extracted the pigment from the insects and added honey and gum arabic to make watercolour.
Violet: Cochineal Cochineal is a bright scarlet insect lake pigment that has been used for centuries to dye textiles, drugs, food and cosmetics. A lake pigment is a pigment made by precipitating a dye with a mordant. Unlike mineral pigments, lake pigments are organic.Cochineal is the result of harvesting the female cochineal parasitic insect that live on the cacti native to Mexico, Central and South America. Using soda ash and alum, I extracted the pigment from the insects and added honey and gum arabic to make watercolour.
Follow Polly on Instagram.
 Close
Close
















































