Success rates
By zceie01, on 1 June 2014
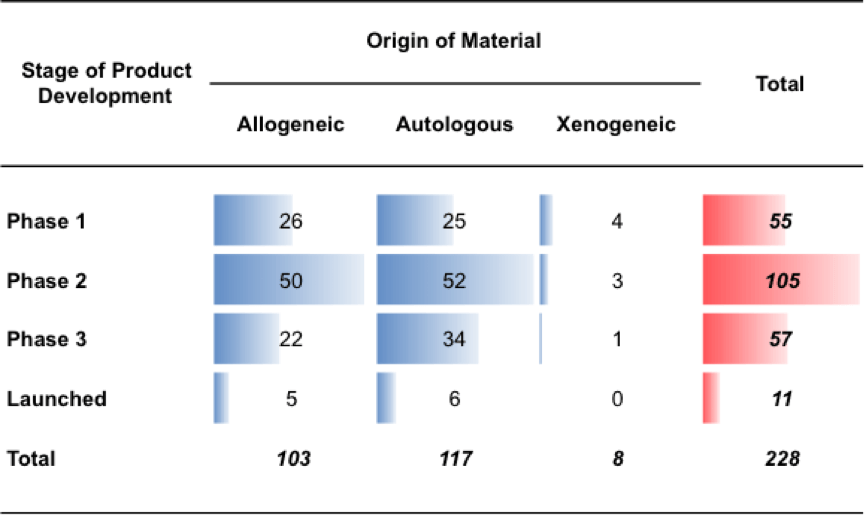
Based on information users provide on the type of projects they are working on (i.e. type of source material, therapeutic focus), the BRITS tool provides estimates of these projects’ likelihood of failure based on failure rates of comparable projects. The BRITS team collected data on these failure rates by examining how many of the projects in the dataset that progressed to a specific development stage (i.e. phase 1,2,3, launch), also progressed to the subsequent development stage, and which projects did not progress. Figure 8 provides a breakdown of the most advanced stages of development different types of cell therapy projects reached.
Figure 8: The most advanced development stage reached by cell therapy projects
Figure 9 provides a breakdown of the success rates of cell therapy projects across various stages of the development process. The BRITS team calculated two different success rates for cell therapy projects across the stages of the product development pathway. First, the BRITS team calculated the success rate as a percentage of projects that started, and did not fail during clinical testing at a specific stage. Second, we calculated the success rate as a percentage of projects that were successful at a specific stage and progressed to the next stage. BRITS data highlight that cell therapy projects tend to have higher success rates than generic therapeutic product development projects, for which success rates are provided in the right-hand column of figure 9.
Figure 9: Success rates of cell therapy projects
77 Responses to “Success rates”
- 1
-
2
Aspire Bedding wrote on 12 May 2023:
With the beautiful bridal bed sheets in Pakistan from Aspire Bedding, you can turn your bedroom into a luxurious heaven. Crafted from premium materials and adorned with intricate designs, these bed sheets will add a touch of elegance to any bedroom decor. Shop now and indulge in the ultimate comfort and style with Aspire Bedding’s bridal bed sheets.
-
3
Mannat Clothing wrote on 12 May 2023:
Looking for some new clothes but don’t want to spend a lot of money? Check out Mannat Clothing’s sale on brands in Pakistan! You’ll find great deals on women’s clothing, including branded suits and dresses. So, take advantage of these discounts and update your wardrobe today!
-
4
Panache Apparel wrote on 12 May 2023:
Panache Apparel is the ultimate destination for stylish western wear, offering a wide range of contemporary and traditional clothing that combines fashion with rugged authenticity.
-
5
Qhaaf Bedding wrote on 13 May 2023:
Take advantage of Qhaaf Bedding’s bedsheet sale, featuring a fantastic collection of premium bed sheets sale online in Pakistan, perfect for upgrading your sleep experience.
- 6
-
7
novelwebcreation wrote on 1 August 2023:
Thank you very much for sharing good content. Useful blog information, wanted to thank you for this excellent read!! Keep it up!
-
8
Rahul Shastri wrote on 1 August 2023:
Thank you for your post, I look for such article along time, today I find it finally.
Vashikaran Specialist Near Me -
9
George Leo wrote on 5 October 2023:
Consider this: why stress when you have a trusted ally in take my proctoru exam for me? Our credentialed team is more than just a service; it’s your ticket to achieving those elusive grades. Leave behind the anxiety, embrace our expertise, and set yourself on a path towards undeniable success.
-
10
Pak Print Wishes wrote on 20 October 2023:
Pak Print Wishes an Best Online Printing Company truly stands out with its unique and creative printing and packaging solutions. They’re your one-stop destination for turning printing needs into exceptional, eye-catching results!
-
11
yati singhal wrote on 8 November 2023:
Learning about success rates is so inspiring! It’s a reminder that with determination and hard work, we can achieve our goals and make our dreams a reality. #SuccessStories
-
12
Alicia Romaro wrote on 20 November 2023:
Your post was fantastic! Its valuable content presented in such an engaging manner was truly commendable. Sharing it is a priority, and I’ve already added it to my bookmarks.
fmovies -
13
Galaxy Toyota wrote on 29 November 2023:
Discover automotive excellence at your fingertips! Find unparalleled service and a stunning array of Toyota vehicles at the Toyota showroom Near Me. Experience personalized assistance, explore cutting-edge models, and embrace a seamless car-buying journey. Your dream Toyota is closer than you think—visit the Toyota showroom near you for an unparalleled automotive experience.
-
14
james robert wrote on 1 December 2023:
Fascinating insights into cell therapy success rates! The BRITS tool’s data breakdown offers a comprehensive understanding of project progression and success at various development stages. Valuable information for those navigating the cell therapy landscape
Driving Suspended License Misdemeanor New Jersey -
15
Bolamd Thomas wrote on 13 December 2023:
¡Esta publicación de blog es realmente inspiradora! Me encanta cómo has explicado el tema con tanta claridad y profundidad. Tu estilo de escritura es atractivo, lo que hace que leerlo sea un placer. Sigan con el fantástico trabajo
Santa Fe Escorts -
16
Eden wheeler wrote on 21 December 2023:
Great post! I really enjoyed reading your insights and the way you presented your ideas was clear and engaging. Thank you for sharing your knowledge and perspective with us. QlikView Developer Course
-
17
kennaanna wrote on 4 January 2024:
Thanks for sharing this insightful information on success rates! It’s fascinating to see how the BRITS tool provides estimates based on failure rates of comparable projects. It reminds me of the way the rice purity test measures different experiences and provides a unique perspective on individual experiences and behaviors.
-
18
Bill Wilson wrote on 13 January 2024:
Website development is an essential part of any business, as the majority of clients (about 80%) are now found through various online channels and sources. In other words, not having a website is only viable for small businesses, in small communities, that are aiming for no more than a couple of dozen customers. Even so, such companies could still use their websites to jumpstart a rapid development process that would help them reach beyond their limitations, and so a business website is more or less a must for any company.
https://www.confiduss.com/en/services/corporate/online/web-development/
-
19
Dominique Kassulke wrote on 17 January 2024:
WhatsApp Plus APK is a well-known and beloved messaging app that lets you easily connect with your loved ones, friends, and colleagues.
-
20
adblocker chrome extension wrote on 19 January 2024:
The true adblocker chrome extension is an essential browser enhancement that transforms your online experience by effectively removing all unwanted ads and empowers you to take control of your browsing domain which provides a clean and distraction-free online journey.
- 21
-
22
Mary Taylor wrote on 19 January 2024:
Well, Now you need not to worry about your pending assignment writing. Best assignment help in UK. We are here to give you best assignment help UK based service. Browse our services of dissertation help london and across UK.
- 23
- 24
-
25
wedding venues in GT Karnal Road wrote on 13 February 2024:
Wedding Venues In GT Karnal Road Book Farmhouses, Banquet Halls, Hotels for Party places at GT Karnal Road Ever thought of enjoying a multi-theme Wedding Functie destination? If non while being at just one destination? If no then you must not have visited Farmhouses.
-
26
Nursing admission essay help wrote on 13 February 2024:
I want to always read your blogs. I love them Are you also searching for Nursing admission essay help? we are the best solution for you. We are best solution for you.
-
27
Nursing Writing Center wrote on 13 February 2024:
I want to always read your blogs. I love them Are you also searching for Nursing Dissertation Writing Help? we are the best solution for you.
-
28
linher wrote on 16 February 2024:
Let your imagination run wild in Quick Draw , the lively party game that encourages creative expression and quick thinking. From abstract shapes to recognizable objects, you’ll be asked to sketch various prompts in 60 seconds or less. It’s a race against the clock, so make sure your pen is ready to bring your artistic ideas to life!
-
29
sfaas wrote on 19 February 2024:
Wenn Sie gerne Sexfilme sehen, sind Sie auf unserer Seite herzlich willkommen. milf pornos
-
30
Abhishek Prabhakar wrote on 20 February 2024:
The Double Declining Balance Depreciation Calculator efficiently computes asset depreciation using the accelerated depreciation method, reflecting higher depreciation expenses in the earlier years of an asset’s life. It offers precise calculations based on initial asset cost, salvage value, and useful life, aiding in accurate financial planning and reporting.
-
31
alexjhon wrote on 25 February 2024:
Thanks a lot for sharing this information with us. I will share this information with my colleagues that are working with me on a project that is related to business chauffeur services.
-
32
DJ Light PK wrote on 27 February 2024:
Shop the best dj disco lights price in Pakistan. We cover all kinds of Party Lights especially for night owls, who loves to dance and party all night. we have unique disco lights, standing corner lamps and smoke machine & a lot more.
-
33
Mafia wrote on 27 February 2024:
Unlock the doors to seamless exploration with Monthly Car Rental Dubai. Your gateway to unparalleled mobility in the city of opulence. ✨ Tailoring to your travel needs, we offer a diverse fleet from economical to luxurious rides.
-
34
Mafia wrote on 27 February 2024:
Unlock the doors to seamless exploration with Monthly car Rental Dubai. Your gateway to unparalleled mobility in the city of opulence. Tailoring to your travel needs, we offer a diverse fleet from economical to luxurious rides.
-
35
MBWhatsapp APK wrote on 1 March 2024:
Upgrade WhatsApp chatting with MBWhatsApp APK Download. Get cool privacy tools, unlimited themes, scheduled texts, anti-revoke and more awesome features. Latest version for Android/iOS for MBios. Customize to next level! Easy download.MB WhatsApp IOS
-
36
onlinecasino wrote on 5 March 2024:
You seem to know how to organize and write neatly. I think this method should be a role model for many people and needs to be learned. I got so much inspiration from just this article and I think I’ll visit often because it will remind me of it sometimes.온라인카지노 I’ll be back to watch this article again next time!
-
37
YouTube ReVanced wrote on 10 March 2024:
Reklamsız en iyi YouTube modu YouTube ReVanced APK ‘dır. İndirme yeteneklerine, Sponsor Engellemeye, geriye doğru uyumsuz oynatmaya ve daha fazlasına sahip olun.
- 38
-
39
Portfolio management tools wrote on 12 March 2024:
When exploring alternatives to Monday.com, Celoxis emerges as the optimal choice, transcending traditional alternatives with its robust features and intuitive interface. Celoxis offers a comprehensive suite of project management tools, seamlessly replacing Monday.com with enhanced functionalities for task management, resource allocation, budget tracking, and reporting. Its user-friendly design ensures a smooth transition while empowering teams to streamline workflows and collaborate effectively. Celoxis stands out as the top pick among alternatives to Monday, enabling organizations to achieve project success with unparalleled ease and efficiency, making it the preferred solution for those seeking a superior project management experience.
-
40
BSNS Consulting wrote on 13 March 2024:
As a leading provider of Business Consultancy Services in Pakistan, BSNS Consulting is your trusted partner for achieving sustainable growth and success. We understand the local business landscape and offer tailored solutions to address your specific challenges. From market analysis to strategic planning, our consultancy services are designed to unlock your business’s full potential.
-
41
weshape soul wrote on 15 March 2024:
weshapesoul.com – Fitness Experts, Nutritionist, Diet Plans, Weight Loss, Health, Wellness, Weight Gain, Dietitian, Fitness, 15 Weight Loss Tips for People With Chronic Condition.
-
42
Hosakebken wrote on 16 March 2024:
Pretty component to content. I simply stumbled upon your weblog and in accession capital to claim that I get actually loved account your blog posts. Any way I will be subscribing in your feeds and even I success you access constantly fast.
-
43
Kwabena wrote on 16 March 2024:
Have you ever considered about including a little bit more than just your articles? I mean, what you say is fundamental and everything. However imagine if you added some great images or videos to give your posts more, “pop”! Your content is excellent but with images and clips, this website could definitely be one of the most beneficial in its niche. Very good blog!
-
44
Kebkengarfiel wrote on 16 March 2024:
Howdy! This is kind of off topic but I need some help from an established blog.
Is it tough to set up your own blog? I’m not very techincal but I can figure things out pretty quick. I’m thinking about making my own but I’m not sure where to start. Do you have any tips or suggestions? Cheers -
45
Richard William wrote on 20 March 2024:
It’s interesting to see the success rates of cell therapy projects and how they compare to generic therapeutic product development projects. The BRITS tool seems to provide valuable insights into the likelihood of failure for different types of projects. On a related note, if you’re interested in discussing this topic further or just want to connect with others in the field, you should check out Omegle
-
46
Centa wrote on 20 March 2024:
Stay Connected, Stay Competitive – Sportzfy APK Keeps You in the Game.
-
47
TipTop Enrichtung wrote on 21 March 2024:
A Barbershop Bedienplatz is the workspace within a barbershop where haircuts, shaving, and other services for male clients are carried out. This area typically includes a chair, mirror, and tools.The Barbershop Service Station is the heart of every barbershop, where skilled barbers work their magic to create fresh looks and maintain well-groomed appearances. This dedicated area is thoughtfully designed for both the barber’s convenience and the client’s comfort.
-
48
Lezty wrote on 21 March 2024:
Das Fahrradfahren ist in Deutschland eine beliebte Art der Fortbewegung. Es gibt viele spezielle Fahrradwege in den Städten und entlang beliebter Radwege in ländlichen Gebieten. Fahrradverleihsysteme sind weit verbreitet, und in einigen Städten findet man sogar gemeinsam genutzte Lastenräder – eine beliebte Art, sich mit Kindern fortzubewegen. Man sollte immer vorsichtig sein und das Fahrrad abschließen, wenn man es unbeaufsichtigt lässt (viapai.com
besonders in Berlin). Autofahrer sind sich der Radfahrer in der Regel bewusst und fahren vorsichtig, dennoch sollte man immer aufmerksam bleiben. Viele Menschen in städtischen Gebieten tragen einen Helm, obwohl dies nicht gesetzlich vorgeschrieben ist. - 49
-
50
Jones elizebeth wrote on 23 March 2024:
Great job for publishing such a beneficial web site. Your web log isn’t only useful but it is additionally really creative too. There tend to be not many people who can certainly write not so simple posts that artistically. Continue the nice writing Johnathan
-
51
Mike wrote on 23 March 2024:
Good composed article. It will be steady to any individual who uses it, including me. Continue doing what you are doing – can’r hold up to peruse more posts. bridal store
-
52
zab sports wrote on 24 March 2024:
ZAB Sports Apparel specializes in creating top-notch custom sports uniforms for various sports teams, clubs and individuals. Our wide selection includes basketball uniforms, soccer uniforms, soccer uniform kits, american football uniforms. soccer uniforms
-
53
mbwhtspk wrote on 28 March 2024:
MB WhatsApp is free to download for Android and iPhone Upgrade WhatsApp chatting with MB WhatsApp APK Download. Get cool privacy tools, unlimited themes, scheduled texts, anti-revoke and more awesome features. The latest version for Android/iOS for MBios. Customize to the next level! Easy download.https://mbwhtspk.com/
-
54
Elmo Eoin wrote on 1 April 2024:
Valuable info. Fortunate me I found your website unintentionally, and I am shocked why this coincidence did not happened earlier!
I bookmarked it. -
55
Hosa Kebken wrote on 1 April 2024:
You’re so interesting! I do not believe I’ve truly read anything like this before. So wonderful to find another person with some unique thoughts on this issue.
Seriously.. thanks for starting this up. This site is one thing that is required on the internet, someone with some originality! -
56
whatsapp blue apk wrote on 3 April 2024:
You ought to take part in a contest for one of the greatest websites on the internet. I am going to highly recommend this website! https://whatsblueapk.com/
- 57
-
58
المثالي الصقور wrote on 7 April 2024:
مكافحة الحشرات هي عملية مهمة للتحكم في انتشار وتأثير الحشرات الضارة على البيئة والصحة العامة. تتضمن هذه العملية استخدام مجموعة من الأساليب والتقنيات للقضاء على الحشرات وتقليل تأثيرها السلبي.
تعد مكافحة الحشرات ضرورية في مختلف البيئات والقطاعات مثل المنازل والمباني التجارية والمزارع والمصانع والمرافق العامة. تهدف إلى حماية الصحة والسلامة العامة من خلال الحد من انتشار الأمراض المنقولة بواسطة الحشرات، فضلاً عن الحد من التلف الذي يمكن أن تسببه الحشرات في الممتلكات والمحاصيل الزراعية.
تشمل طرق مكافحة الحشرات استخدام المبيدات الحشرية لقتل الحشرات أو التحكم في تكاثرها، واستخدام الأفخاخ والشباك الحاجزة لمنع دخول الحشرات إلى المناطق المحمية، وتطبيق تقنيات التحكم البيولوجي التي تعتمد على استخدام الكائنات الحية المفيدة للقضاء على الحشرات الضارة.
.
.
شركة مكافحة حشرات -
59
john david wrote on 7 April 2024:
Photoleap MOD APK lets you edit photos like a pro! Download it to get awesome filters, effects, and cool AI tools for free. Make your pics look amazing with easy editing.
-
60
john david wrote on 7 April 2024:
Pikashow APK offers HD streaming of entertainment. Includes TV shows and movies. Download the latest version for Android, PC or iOS.
-
61
john david wrote on 7 April 2024:
IGram on Download Instagram videos easily with IGram. This free online tool lets you save Instagram video posts directly to your device.
-
62
calculator gpa wrote on 8 April 2024:
Your GPA is easily calculated by using our GPA Calculator, gpa calculator along with the achieved grade and credits for each course.
- 63
-
64
https://mbwhtspk.com/how-to-install-mb-whatsapp-on-windows-and-pc/ wrote on 8 April 2024:
In this modern era, we mostly spend our time working on PC and Windows; therefore, it’s quite annoying to check your phone repeatedly to chat with friends.
-
65
Arham khan wrote on 8 April 2024:
Gb WhatsApp offers enhanced features beyond the standard WhatsApp, including customization options, privacy controls, and additional security measures. With Gb WhatsApp(https://gbwhpro.com/gbwhatsapp-5-90-apk/), users can enjoy more flexibility in messaging, such as scheduling messages, hiding online status, and accessing a wider range of emojis and themes. It provides a seamless and enriched communication experience for WhatsApp users.
-
66
Printable quotes wrote on 18 April 2024:
Printable quotes are a fantastic way to add a touch of inspiration and motivation to your surroundings. Whether you’re looking to decorate your workspace, bedroom, or any other area, printable quotes offer a simple yet impactful way to infuse positivity into your environment. https://printaboles.com
-
67
Huanra wrote on 19 April 2024:
Discover luxurious retreats amidst the picturesque landscapes of el Metn with our exclusive listings of apartments for sale. Immerse yourself in opulence and tranquility as you explore residences that offer unparalleled comfort and breathtaking views, promising an extraordinary living experience, click apartments for sale in el metn
-
68
GB WhatsApp wrote on 22 April 2024:
GBWhatsApp includes features like scheduling messages, automatically replying to messages, and hiding chats.
Dual WhatsApp: GBWhatsApp allows users to run two WhatsApp accounts simultaneously on the same device.https://gbwhatsapppro-in.com/ -
69
Gold WhatsApp wrote on 22 April 2024:
“Gold WhatsApp” is a newer development or a niche version of the app that has emerged since my last update, I wouldn’t have information on it. It’s always a good idea to research and understand the potential risks before using any unofficial version of WhatsApp or any other app.
Gold WhatsApp APK Download -
70
jekure wrote on 24 April 2024:
By promoting wireless file sharing, xender.one contributes to reducing the environmental footprint associated with traditional file transfer methods involving physical media.
- 71
-
72
MB WhatsApp wrote on 29 April 2024:
“MB WhatsApp offers enhanced features and customization options, providing users with a tailored messaging experience. It is a modified version of WhatsApp, catering to those seeking additional functionalities and personalization.”https://thegbapk.com/mb-whatsapp-ios/
-
73
apps 2024 xender wrote on 7 May 2024:
Seamless Sharing with XenderApp: Anytime, Anywhere.
https://xenderapp.me/ -
74
james1165 wrote on 7 May 2024:
Online assignment help services provide valuable academic support to students. These services offer expert guidance, research assistance, and writing support across various subjects and topics. They help students understand assignment requirements, improve their writing skills, and achieve better grades by delivering high-quality and well-researched content tailored to individual needs.
-
75
james1165 wrote on 8 May 2024:
Online assignment help provides academic assistance to students through virtual platforms. It offers services like essay writing, homework support, and project guidance across various subjects. Expert tutors ensure timely delivery, plagiarism-free content, and customized solutions to meet students’ academic needs effectively.
-
76
lily lily wrote on 9 May 2024:
The BRITS That’s Not My Neighbor tool provides clear insight into the likelihood of project failure based on a variety of factors such as raw material source and treatment focus.
-
77
delwy charmaine wrote on 9 May 2024:
I love how fnaf combines gameplay and story elements, creating a complete and enjoyable experience from start to finish.
 Close
Close



Is it useful?