In the first interview as part of the new Early Career Innovators series, acknowledging the amazing translational work being done by early career researchers within the UCL Therapeutic Innovation Networks, Dr Giulia De Rossi highlights her Biologics TIN Pilot Data Fund awarded project “LRG1 antibody for diabetic macular oedema” and presents some advice for future applicants.
Please provide an overview of your Biologics project.
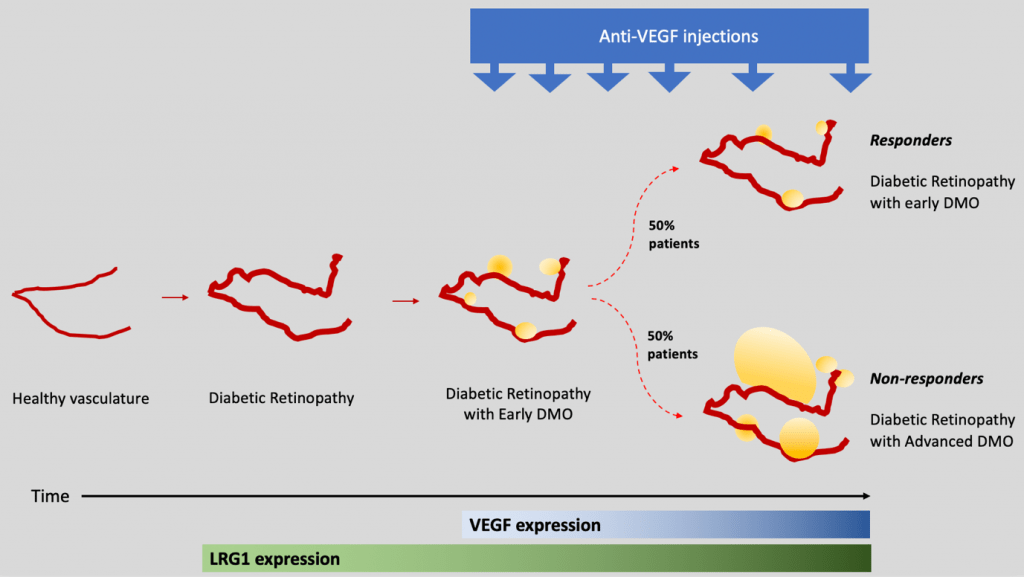
There are currently 4.8 million people in the UK living with diabetes and over 300,000 of these have their sight threatened by a severe ocular complication called diabetic macular oedema (DMO), which has now become the most common cause of blindness in the working population.
Clinical studies have shown that leucine-rich alpha-2-glycoprotein 1 (LRG1) is enriched in the eyes of diabetic patients and we showed in other settings that it can drive vascular dysfunction. My hypothesis is that LRG1 is an early pathological switch in DMO and I believe that it may represent a novel/alternative pathway we could target therapeutically.
My project “LRG1 antibody for diabetic macular oedema” will test the efficacy of function-blocking antibody against LRG1 using murine models of diabetes. Specifically, I will be looking at the effects of this biologic on vascular homeostasis and permeability.

3D Reconstruction of the retinal vasculature.
What is the motivation behind your project?
Currently, if you are diagnosed with DMO, you will receive monthly intra-ocular injections of VEGF-neutralizing antibodies. Unfortunately, this line of treatment has only a 50% chance of working and often responses are short-lived. As a result, despite the NHS spending £116m/annum, 2000 people go blind every year.
With diabetes reaching epidemic proportions, there is an urgent unmet need and market for developing new treatments for this devastating condition.
I believe targeting LRG1 with a function-blocking antibody has the potential not only to treat patients who are refractory to current therapies, but also to achieve earlier and therefore better outcomes in all patients.
Why did you want to apply to the Biologics TIN Pilot Data Fund?
I had an interesting set of preliminary data that I felt was suitable for applying for a pilot proof-of-concept grant.
The TIN pilot data fund was also my first opportunity to apply for and manage a grant as the lead applicant, which I hope will be a first stepping stone towards independent research and career development.
What do you hope to achieve in the 6 months duration of your project?
With the funding requested I plan to answer 3 critical questions: 1) Is LRG1 a pathological switch in DMO? 2) Is a Lrg1-deficient mouse protected from DMO? and 3) Can anti-LRG1 antibodies prevent the onset of DMO?
What are your next steps from now?
My planned experiments will support the preclinical dataset necessary to take the anti-LRG1 antibody into clinical trials, by establishing whether LRG1 is a valid target, and, together with the available supporting literature from clinical studies, will constitute the foundation for a translational grant application next year.
Do you have any top-tips for applicants currently going through the application process for the other TIN Pilot Data Funds?
Make sure you clearly describe: what the problem is, your proposed solution, why your approach is better, what you want to do next if successful (clear career and translational path).
As with every application with a limited word count, you might end up with an extremely abridged version of your initial text and some key concepts might become cryptic. Have someone from a different field review your application and tell you if they can still understand the key message you want to convey.
There is no space to describe the experiments in detail, so just explain the scientific questions you want to answer, you will have the opportunity to wear your scientist hat if you get to the Q&A stage.
Good luck future applicants!
Dr Giulia De Rossi will be discussing her Biologics TIN Pilot Data Scheme application process experience in the upcoming ACCELERATE Success event, “Grant Writing for Translational Research” on Tuesday 6th October. This is an educational, translational training event to help UCL researchers write impactful applications by recognising the important elements of a translational research/innovation grant application and increase chances of funding success. Register here.
About Dr Giulia De Rossi

Dr Giulia De Rossi is a research fellow at the UCL Institute of Ophthalmology in the lab led by Professor Stephen Moss and Professor John Greenwood. Dr De Rossi’s training was in Biotechnology and the main focus of her PhD and post-doctoral work has been understanding the mechanisms underpinning vascular dysfunction and new blood vessel formation.
Dr De Rossi joined UCL in July 2019 to work on a Diabetes UK-funded project aimed at identifying new targets and mechanisms to treat the microvascular complications of diabetes.
 Close
Close





