The Mystery of Iridescence in Glass
By Anna Pokorska, on 20 May 2019
This is the second part on a series on ‘Iridescence’. You can read the first part here, or return and read an introduction to colours, as well as individually about the colours blue, red, yellow, and green.
If you’ve ever wandered through a museum displaying ancient artefacts, chances are you were amazed at the quality and artistry displayed in glass objects of that time. The has some incredible pieces shining with iridescent colours:
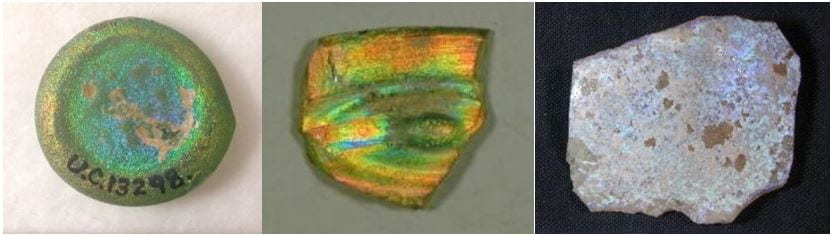
Left: glass weight from the Fatimid period; Middle: glass fragment from the Roman period, possibly part of an eye amulet; Right: glass fragment from the late Roman period (Petrie Museum: UC13298, UC22744, UC67914).
However, despite the undeniable talents of ancient glassmakers, this particular effect was not intentional or even achieved during production. In fact, iridescence found in ancient glass is a result of weathering of its surface caused by burial. The weathering process itself depends largely on the burial conditions such as heat, humidity and type of soil, although the chemistry of the glass, determined by the purity of raw materials and their compositional ratio, also plays a part. The iridescence is produced when alkalis, or soluble salts, are leached from the buried glass by slightly acidic water present in the soil. This in turn causes the formation of very fine layers which can delaminate or even flake off creating a prism effect.
But it wasn’t until the very end of the 19th century that the iridescence of ancient glass was replicated by Louis Comfort Tiffany (1848-1933), the son of Charles Tiffany – the New York jeweller. He began his career as an aspiring painter but soon realised that his true potential was in interior decoration. It is generally thought that during his extensive travels Tiffany became inspired by the glasswork and mosaics of antiquity and devoted to the idea of restoring stained glass to its former glory by striving to achieve the same standards of beauty as the ones present in antique masterpieces[1]. Prior to the twelfth century, stained glass works were executed with differently coloured glass pieces as opposed to the later technique of painting on clear glass, which dulled it considerably and created a flat two-dimensional effect. Tiffany’s experiments with glass during the 1880s completely revolutionized the look of the medium and in 1894 he patented favrile glass[2]. By adding different or same shades of colour into the hot mixture Tiffany created a material different from other iridescent glasses as the effect was not just confined to the surface but part of the glass itself.
Tiffany Glass and Decorating Company was established in 1892 in New York and began producing its first favrile glass objects 1896, examples of which can be found in the Victoria and Albert Museum collection as well as other major museums, particularly in America.

Favrile glass objects produced by the Tiffany Glass and Decorating Company between 1896 and 1902 (Image: © Victoria and Albert Museum, London).

Left: “The Flight of Souls”, Tiffany stained glass window which won first prize at the 1900 Paris Exposition, now at the Wade Memorial Chapel, Cleveland, Ohio (Image: CoffeeDoc03); Right: Hanging Head Dragonfly Tiffany lamp from the Art Institute of Chicago collection (Image: mark6mauno).
Tiffany won first prize for the above stained-glass window using his new material at the 1900 Paris Exposition and continued to use favrile for other products, including his famous lamps. Being the innovator that he was, he also carried on experimenting with the medium, eventually developing many other, equally impressive, types of glass such as opalescent, streamer, fracture, ring-mottle, ripple and drapery. But that’s for another time!
[1] Bing, S Louis C. Tiffany’s Coloured Glass Work, in Artistic America, Tiffany glass and Art Nouveau, Cambridge (Mass.); London: M.I.T. Press, 1970
[2] The original trade name was actually fabrile, which was derived from an Old English word meaning ‘handcrafted’.
 Close
Close





