Measure Twice, Cut Once:
Quantifying Biases in Sexual Selection Studies
By Claire Asher, on 25 June 2014
Bateman’s principles are conceptually quite simple, but form the basis of our understanding of sexual selection across the animal kingdom. First proposed in 1948, Bateman’s three principles posit that sexual selection is more intense in males than in females for three reasons:
1) males show more variability in the number of mates they have (mating success);
2) males show more variability in the number of offspring they have (reproductive success);
3) the slope of the relationship between mating and reproductive success is steeper in males;
Together, this summarises our basic view of sexual selection in the majority of sexually reproducing species – males that do well, do very well and we expect more intense sexual selection because of it.
Biased Traditions
Traditionally, most studies investigating these relationships have measured mating success by counting the number of females a male produces offspring with. This method is biased though, as it assumes that every mating results in offspring, which is unlikely to be true. Further, it assumes that every fertilisation produces an offspring, which ignores cases where embryos die before birth. Using offspring counts as a way to measure mating success might not be accurate but it is certainly more practical – behavioural observations of actual mating would be very time consuming and nearly impossible for some species. However, until now no study has attempted to quantify the importance of these biases in calculating and testing Bateman’s principles.
Carefully Observed
To address this issue, GEE researchers Dr Julie Collet and Dr Rebecca Dean, in collaboration with researchers at the University of Oxford, University of Queensland, Uppsala University and the University of East Anglia, investigated mating and reproductive success in Red Junglefowl (Gallus gallus). They recorded matings and collected all eggs laid from 13 groupings of 3 males and 4 females (mimicking natural conditions). They began by using classic techniques to estimate Bateman’s gradients – they inferred mating success from the number of females they sired an offspring with. They found twice as much variability in male mating success, and four times as much variance in male reproductive success (the actual number of offspring a male produced) compared with females. Mating success and reproductive success were strongly related – differences between individuals in mating success explained 57% of variance in reproductive success in males, but only 24% in females, and the slope of the relationship was steeper in males.
Covarying Factors
Reproductive success is not just a product of how many mates you have. The fecundity of your mate is also a crucial factor, and in species where females mate with multiple males, your share of her offspring is also a key variable. The authors investigated whether these variables tend to be related and whether multivariate analyses that take them all into account better explain the overall reproductive success of a male. Their multivariate model explained the variance in male mating success better than the standard approach and found that mating success, paternity share and mate fecundity together are responsible for the variance in male reproductive success. The authors estimate that by ignoring these other factors, other studies may overestimate the Bateman gradient by as much as 150%!
This study shows the importance of investigating the biases we introduce into our science. These biases may sometimes be inevitable, if excluding them is extremely time consuming or difficult. But we must try to understand the influence of these biases in order to draw informed conclusions from our data. Here, GEE researchers demonstrate how using biased measures of mating success can cause scientists to overestimate the opportunity for sexual selection on males. This effect is likely to be largest for species which have small clutch sizes and in which sperm competition plays a key role. Where possible, studies investigating sexual selection should include accurate measures of mating success, and include other variables such as paternity share and mate fecundity in a multivariate approach in order to best understand Bateman’s principles and the relationship between mating and reproductive success in both sexes.
Original Article:
This research was made possible by funding from the Natural Environment Research Council (NERC), the Biotechnology and Biological Sciences Research Council (BBSRC) and Marie Curie Action.
 Close
Close








 So, UPI makes a lot of sense, evolutionarily, and some scientists think it might also explain why we have two sexes, as opposed to any other mating system. It’s important to be clear, when we talk about having two sexes we’re saying nothing about the external differences between the sexes (sexual dimorphism) observed in many multicellular organisms. We’re talking about the existence of two ‘mating types’, such that individuals cannot mate with members of the same type. Recent research by another group of GEE academics including Professor Andrew Pomiankowski, Dr Nick Lane and Professor Robert Seymour, investigated the evolution of UPI and in particular it’s relationship with the evolution of a two-sex mating system. We might expect a strong link between UPI and the existence of two sexes, since uniparental inheritance immediately generates differences between the two mating partners, and ensures that reproduction is not possible unless one member of each ‘type’ is present. Although UPI is often thought to have been a key driver in the evolution of mating types, there have been few investigations of what conditions are needed for the fitness benefits of UPI to actively drive the emergence of two mating types. So the authors developed a new mathematical model of mitochondrial inheritance and the evolution of UPI in a population where biparental inheritance (BPI) is the norm. They incorporated mitochondrial mutation (which might sometimes be selfish) and selection into the model, and included different mating types.
So, UPI makes a lot of sense, evolutionarily, and some scientists think it might also explain why we have two sexes, as opposed to any other mating system. It’s important to be clear, when we talk about having two sexes we’re saying nothing about the external differences between the sexes (sexual dimorphism) observed in many multicellular organisms. We’re talking about the existence of two ‘mating types’, such that individuals cannot mate with members of the same type. Recent research by another group of GEE academics including Professor Andrew Pomiankowski, Dr Nick Lane and Professor Robert Seymour, investigated the evolution of UPI and in particular it’s relationship with the evolution of a two-sex mating system. We might expect a strong link between UPI and the existence of two sexes, since uniparental inheritance immediately generates differences between the two mating partners, and ensures that reproduction is not possible unless one member of each ‘type’ is present. Although UPI is often thought to have been a key driver in the evolution of mating types, there have been few investigations of what conditions are needed for the fitness benefits of UPI to actively drive the emergence of two mating types. So the authors developed a new mathematical model of mitochondrial inheritance and the evolution of UPI in a population where biparental inheritance (BPI) is the norm. They incorporated mitochondrial mutation (which might sometimes be selfish) and selection into the model, and included different mating types. 



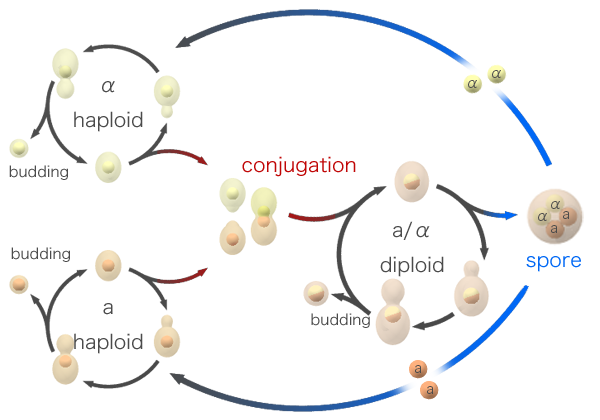 Yeast, being single-celled, are able to reproduce asexually – budding off a new copy of themselves. However, when times are hard, yeast can also enter a sexual phase of reproduction. When nutrient levels are low, a yeast cell will divide into sexual spores. These spores are haploid, carrying only one copy of each chromosome (rather than the usual two), making them analogous to sperm and egg cells. Spores will remain dormant until they sense an improvement in the environment, at which point they spring into action and try to find a mate. Spores come in two mating types and must always mate with another spore of the opposite type – a system based on mating pheromones released by spores to attract the opposite sex.
Yeast, being single-celled, are able to reproduce asexually – budding off a new copy of themselves. However, when times are hard, yeast can also enter a sexual phase of reproduction. When nutrient levels are low, a yeast cell will divide into sexual spores. These spores are haploid, carrying only one copy of each chromosome (rather than the usual two), making them analogous to sperm and egg cells. Spores will remain dormant until they sense an improvement in the environment, at which point they spring into action and try to find a mate. Spores come in two mating types and must always mate with another spore of the opposite type – a system based on mating pheromones released by spores to attract the opposite sex.

 The huge diversity of placental mammals on Earth today first appeared shortly after the mass extinction event that killed the dinosaurs. It is thought that the loss of the dinosaurs, along with much of life on Earth, freed up niches which placental mammals to evolved to fill. But were early placental mammals present, waiting in the wings, during the age of the dinosaurs, or did they appear rapidly after their demise? One recent study suggested that, based on fossil evidence, the placental mammals must have appeared after the cretaceous-tertiary boundary (KT) when dinosaurs and most life on Earth was wiped out. However, a recent paper by GEE’s Mario dos Reis and Ziheng Yang, in collaboration with Philip Donoghue from the University of Bristol, highlights flaws in the methods used in this study, and utilitsed a more thorough approach to show that early placental mammals likely predated the KT boundary.
The huge diversity of placental mammals on Earth today first appeared shortly after the mass extinction event that killed the dinosaurs. It is thought that the loss of the dinosaurs, along with much of life on Earth, freed up niches which placental mammals to evolved to fill. But were early placental mammals present, waiting in the wings, during the age of the dinosaurs, or did they appear rapidly after their demise? One recent study suggested that, based on fossil evidence, the placental mammals must have appeared after the cretaceous-tertiary boundary (KT) when dinosaurs and most life on Earth was wiped out. However, a recent paper by GEE’s Mario dos Reis and Ziheng Yang, in collaboration with Philip Donoghue from the University of Bristol, highlights flaws in the methods used in this study, and utilitsed a more thorough approach to show that early placental mammals likely predated the KT boundary.


 Dr Kivell (University of Kent) and UCL’s Anna Barros and Dr Smaers, compared wrist bone features across 24 living primate species and 16 extinct species. Primate wrists are composed of between 8 and 9 separate bones, and they discovered differing evolutionary patterns for different bones, indicating that each bone evolves at least partly independently from the others. Some of the evolutionary changes that occurred during primate evolution are shared between species which move in similar ways, whilst others are shared between closely related species, regardless of locomotion. Hominids tended to show more morphological variation than monkeys, suggesting stronger selection on the hominid wrist, possible relating to rapid and major changes in body size and locomotion in these species.
Dr Kivell (University of Kent) and UCL’s Anna Barros and Dr Smaers, compared wrist bone features across 24 living primate species and 16 extinct species. Primate wrists are composed of between 8 and 9 separate bones, and they discovered differing evolutionary patterns for different bones, indicating that each bone evolves at least partly independently from the others. Some of the evolutionary changes that occurred during primate evolution are shared between species which move in similar ways, whilst others are shared between closely related species, regardless of locomotion. Hominids tended to show more morphological variation than monkeys, suggesting stronger selection on the hominid wrist, possible relating to rapid and major changes in body size and locomotion in these species.