It Pays to Be Different:
Evolutionary Distinctiveness and Conservation Priorities
By Claire Asher, on 15 July 2014
The world is currently experiencing an extinction crisis. A mass extinction on a scale not seen since the dinosaurs. While conservationists work tirelessly to try and protect the World’s biodiversity, it will not be possible to save everything, and it is important to focus conservation efforts intelligently. Evolutionary distinctiveness is a measure of how isolated a species is on it’s family tree – how long ago it split off from its nearest living relative. A recent paper co-authored by UCL GEE’s Dr David Redding, published in Current Biology, assessed how effective evolutionary distinctiveness is a tool for identifying bird species of conservation priority. Current conservation efforts are missing some of the most evolutionarily distinct species.
Evolutionary distinctiveness (ED) is measured as the distance along the evolutionary tree from one species to it’s nearest relative. It can be used as a measure of how much evolutionary ‘information’ would be lost if this species were to become extinct. We have good estimates of these distances for birds as we have been able to put dates on the evolutionary tree based on fossil records and molecular data. A recent analysis of nearly 10,000 known bird species, by researchers at Yale University, Imperial College London, University of Sheffield, Simon Fraser University, University of Tasmania and University College London, showed some patterns we might have expected, for example, evolutionary distinctiveness is highest in isolated regions (e.g. Australia, New Zealand and Madagascar) and regions with higher species richness tended to have more evolutionary distinct birds. However, there were also some unexpected results. For example, ED wasn’t strongly related to latitude, a pattern predicted by the idea that the tropics act as a ‘museum’ for ancient lineages, nor was ED related to a species’ range-size, which has previously been predicted theoretically.
Evolutionary distinctiveness showed little relationship with conservation status – some of the most threatened distinct species are found outside of biodiverse regions that are usually the target of conservation efforts. This means that, when we consider only species richness or total biodiversity to identify regions to conserve, we may be missing a great deal of evolutionary information. Instead, basing areas of conservation priority on the evolutionary distinctiveness of their flora and fauna may offer a more efficient and effective way to maximise the evolutionary variation we keep.
The paper also released the first formal list of ‘EDGE birds’ – EDGE stands for “Evolutionary Distinctive and Globally Endangered” and is a metric combining ED with the IUCN Red List. The list includes the Giant Ibis, the New Caledonian Owlet-Nightjar, the California Condor, the Kakapo, the Philippine Eagle, the Christmas Island Frigatebird and the Kagu, all of which are listed as either Critically Endangered or Endangered.
The most evolutionary distinct birds include both common species and rare species, both isolated and wildly distributed species, and are found in almost every environment on Earth. Current conservation efforts that focus on tropical regions with high species richness may be neglecting many evolutionary distinct species, whose extinction would represent the loss of a great deal of ‘evolutionary information’. Evolutionary distinctiveness could offer a powerful tool to supplement current criteria for identifying conservation priorities.
Original Article:
This research was made possible by funding from the Natural Environment Research Council (NERC), the Natural Sciences and Engineering Research Council (NSERC), the National Science Foundation, and the National Aeronautics and Space Administration (NASA)
 Close
Close










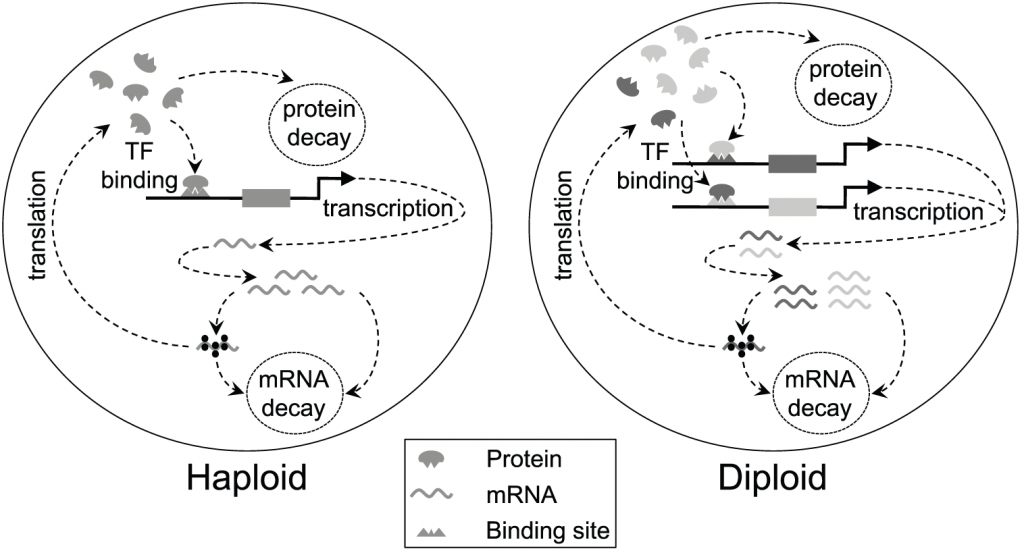
 So, UPI makes a lot of sense, evolutionarily, and some scientists think it might also explain why we have two sexes, as opposed to any other mating system. It’s important to be clear, when we talk about having two sexes we’re saying nothing about the external differences between the sexes (sexual dimorphism) observed in many multicellular organisms. We’re talking about the existence of two ‘mating types’, such that individuals cannot mate with members of the same type. Recent research by another group of GEE academics including Professor Andrew Pomiankowski, Dr Nick Lane and Professor Robert Seymour, investigated the evolution of UPI and in particular it’s relationship with the evolution of a two-sex mating system. We might expect a strong link between UPI and the existence of two sexes, since uniparental inheritance immediately generates differences between the two mating partners, and ensures that reproduction is not possible unless one member of each ‘type’ is present. Although UPI is often thought to have been a key driver in the evolution of mating types, there have been few investigations of what conditions are needed for the fitness benefits of UPI to actively drive the emergence of two mating types. So the authors developed a new mathematical model of mitochondrial inheritance and the evolution of UPI in a population where biparental inheritance (BPI) is the norm. They incorporated mitochondrial mutation (which might sometimes be selfish) and selection into the model, and included different mating types.
So, UPI makes a lot of sense, evolutionarily, and some scientists think it might also explain why we have two sexes, as opposed to any other mating system. It’s important to be clear, when we talk about having two sexes we’re saying nothing about the external differences between the sexes (sexual dimorphism) observed in many multicellular organisms. We’re talking about the existence of two ‘mating types’, such that individuals cannot mate with members of the same type. Recent research by another group of GEE academics including Professor Andrew Pomiankowski, Dr Nick Lane and Professor Robert Seymour, investigated the evolution of UPI and in particular it’s relationship with the evolution of a two-sex mating system. We might expect a strong link between UPI and the existence of two sexes, since uniparental inheritance immediately generates differences between the two mating partners, and ensures that reproduction is not possible unless one member of each ‘type’ is present. Although UPI is often thought to have been a key driver in the evolution of mating types, there have been few investigations of what conditions are needed for the fitness benefits of UPI to actively drive the emergence of two mating types. So the authors developed a new mathematical model of mitochondrial inheritance and the evolution of UPI in a population where biparental inheritance (BPI) is the norm. They incorporated mitochondrial mutation (which might sometimes be selfish) and selection into the model, and included different mating types. 



 The huge diversity of placental mammals on Earth today first appeared shortly after the mass extinction event that killed the dinosaurs. It is thought that the loss of the dinosaurs, along with much of life on Earth, freed up niches which placental mammals to evolved to fill. But were early placental mammals present, waiting in the wings, during the age of the dinosaurs, or did they appear rapidly after their demise? One recent study suggested that, based on fossil evidence, the placental mammals must have appeared after the cretaceous-tertiary boundary (KT) when dinosaurs and most life on Earth was wiped out. However, a recent paper by GEE’s Mario dos Reis and Ziheng Yang, in collaboration with Philip Donoghue from the University of Bristol, highlights flaws in the methods used in this study, and utilitsed a more thorough approach to show that early placental mammals likely predated the KT boundary.
The huge diversity of placental mammals on Earth today first appeared shortly after the mass extinction event that killed the dinosaurs. It is thought that the loss of the dinosaurs, along with much of life on Earth, freed up niches which placental mammals to evolved to fill. But were early placental mammals present, waiting in the wings, during the age of the dinosaurs, or did they appear rapidly after their demise? One recent study suggested that, based on fossil evidence, the placental mammals must have appeared after the cretaceous-tertiary boundary (KT) when dinosaurs and most life on Earth was wiped out. However, a recent paper by GEE’s Mario dos Reis and Ziheng Yang, in collaboration with Philip Donoghue from the University of Bristol, highlights flaws in the methods used in this study, and utilitsed a more thorough approach to show that early placental mammals likely predated the KT boundary.
 Dr Kivell (University of Kent) and UCL’s Anna Barros and Dr Smaers, compared wrist bone features across 24 living primate species and 16 extinct species. Primate wrists are composed of between 8 and 9 separate bones, and they discovered differing evolutionary patterns for different bones, indicating that each bone evolves at least partly independently from the others. Some of the evolutionary changes that occurred during primate evolution are shared between species which move in similar ways, whilst others are shared between closely related species, regardless of locomotion. Hominids tended to show more morphological variation than monkeys, suggesting stronger selection on the hominid wrist, possible relating to rapid and major changes in body size and locomotion in these species.
Dr Kivell (University of Kent) and UCL’s Anna Barros and Dr Smaers, compared wrist bone features across 24 living primate species and 16 extinct species. Primate wrists are composed of between 8 and 9 separate bones, and they discovered differing evolutionary patterns for different bones, indicating that each bone evolves at least partly independently from the others. Some of the evolutionary changes that occurred during primate evolution are shared between species which move in similar ways, whilst others are shared between closely related species, regardless of locomotion. Hominids tended to show more morphological variation than monkeys, suggesting stronger selection on the hominid wrist, possible relating to rapid and major changes in body size and locomotion in these species.